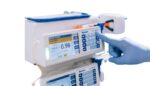
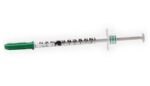
The FDA determined that another recall of BD (NYSE:BDX) Alaris infusion pumps is Class I, the most serious kind. In the latest Alaris recall, the company cites compatibility issues with Cardinal Health Monoject syringes. The issue mainly relates to changes made to the products by Cardinal Health. It affects more than 1 million total devices. […]
Food & Drug Administration (FDA)
Aptar wins FDA contract to study low global warning potential metered dose inhalers
Aptar Pharma announced today that it entered into a contract with the FDA to work on low global warming potential (low-GWP) inhalers. The FDA enlisted Aptar to study the challenges of developing low-GWP propellant metered dose inhalers (MDIs). The Crystal Lake, Illinois-based company expects its study to help define the potential target product profile of […]
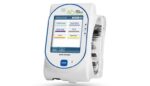
Baxter issues Novum IQ infusion pump warning
Correction: the original version of this article identified this as another recall for Novum IQ. Baxter issued a notification related to a Nov. 15 recall, not a notification for a separate recall. This story has been updated. Baxter (NYSE:BAX) issued an urgent medical device correction notice regarding its Novum IQ syringe infusion pump. A few […]
Eitan Medical’s Sapphire infusion pump recall is Class I
The FDA determined that the recall of certain Eitan Medical Sapphire infusion pumps is Class I, the most serious kind. Netanya, Israel-based Eitan recalled certain Sapphire pumps running software version Rev 16.10. The recall includes the Sapphire multi-therapy pump, the Sapphire Epidural pump and the Sapphire Plus pump. Eitan initiated the recall on Sept. 11, […]
FDA warning doesn’t affect Embecta syringes, company says
Embecta (Nasdaq:EMBC) says an FDA safety communication regarding certain plastic syringes has no impact on its own products. The FDA yesterday warned of the potential for device failures with plastic syringes manufactured in China. Failures could include leaks, breakage and other problems. The agency said it received information about quality issues associated with several Chinese […]
B. Braun infusion pump battery recall is Class I after one reported death
The FDA labeled a recall of B. Braun Medical infusion pump battery packs Class I, the most serious kind. B. Braun’s recall is a correction, not a product removal, affecting the battery pack for its Infusomat Space large-volume pump. It affects both wireless and non-wireless batteries, model numbers 8713051U and 8713052U. According to an FDA […]
Baxter recalls some syringe pumps due to underdosing risk
The FDA determined that the recall of the Baxter (NYSE:BAX) Novum IQ syringe pump is Class 1, the most serious kind. Baxter designed its Novum IQ syringe pump as an infusion pump to deliver fluids into a patient’s body in a controlled manner. The device is suitable for patient care in hospitals and outpatient facilities […]
FDA issues warning about over-the-counter eye drops
The FDA issued a notice warning consumers about 26 over-the-counter eye drop products that could result in eye infections. In its notice, the FDA instructed consumers not to purchase and to immediately stop using these products. Using those eye drops could lead to the risk of infections that could result in partial vision loss or […]
FDA clears iPhone app for the Insulet Omnipod 5
Insulet (Nasdaq:PODD) announced today that the FDA granted 510(k) clearance for its Omnipod 5 App for iPhone. Clearance makes Insulet the only company offering a tubeless, automated insulin delivery (AID) system with full control from a compatible Android and iOS smartphone. The company first launched the system, compatible with Android devices, in August 2022. Insulet […]
FDA approves next-gen intrathecal drug delivery system from Medtronic
Medtronic (NYSE:MDT) announced today that the FDA approved its next-generation SynchroMed III intrathecal drug delivery system. The medtech giant designed SynchroMed III to treat patients with chronic pain, cancer pain and severe spasticity. The targeted drug delivery (TDD) system alleviates symptoms by delivering medication directly to the fluid surrounding the spinal cord. SynchroMed III builds upon […]