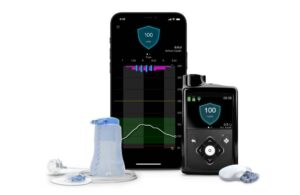
The latest data sets evaluated the recently FDA-approved system across a wide range of users. They looked at younger patients, those not meeting glycemic goals and individuals using simplified meal announcement technology. Medtronic presented these results at the 83rd American Diabetes Association (ADA) Scientific Sessions in San Diego.
Among the findings, Medtronic reported positive results for its proprietary Meal Detection technology. MiniMed 780G is the only system with meal detection technology providing automatic adjustments and corrections to sugar levels every five minutes. This occurs for both basal (background) and bolus (mealtime) insulin needs.
Que Dallara, Medtronic Diabetes EVP and president, previously told Drug Delivery Business News that this feature is “a big deal.”
“If you think about the original concept of the artificial pancreas, it’s the closest you can get to this dynamic closed-loop environment,” Dallara explained.
The simplified meal announcement system leverages fixed carbohydrate amounts instead of exact carb calculations. Medtronic said the studies demonstrated that it supported time-in-range outcomes exceeding consensus guidelines of 70%. Additionally, the studies demonstrated reduced time spent in hyperglycemia for both children and adults.
Medtronic began shipping the next-generation MiniMed 780G system in the U.S. this month.
Study demonstrates Meal Detection technology’s capabilities in adolescents
Dr. Goran Petrovski of Sidra Medicine presented the first study of randomly assigned adolescents using the MiniMed 780G system. With 34 adolescents split into two groups, some entered a fixed number of carbs (small, average or high) and some calculated a precise number for their meals. These individuals lived with diabetes for at least one year and used multiple daily injections or pump therapy prior to the study.
Results demonstrated that those using fixed carb entry maintained international targets for glycemic control over six months. That includes a A1c of 6.9% and time-in-range of 72.7% (compared to 79.4% in the precise entry group). The simplified entry group lowered their time above 250 mg/dL from 28.3% to 5.3% at six months (vs. 3.9% in the precise entry group). After three months, 88% of users chose to continue with the simplified approach, suggesting user satisfaction.
Medtronic said the results suggest that reduced accuracy in carb counting can be offset by the increased automated insulin delivery the system provides. Those that can’t or don’t input carbs precisely can reach glycemic goals and reduce hyperglycemia, Medtronic said.
“Many individuals with type 1 diabetes struggle with meal management with nearly 50% considering carb counting the most burdensome aspect of diabetes management. Indeed, many frequently underestimate their carbs or forget to bolus and this has an adverse impact on clinical outcomes,” said Dr. Petrovski. “This study shows that a simplified meal management approach with the MiniMed 780G system helped users maintain glycemic targets while providing forgiveness for inexact carb counts. Clearly there’s more runway for simplification of diabetes management with this system and it’s promising for the many patients struggling with meal management.”
More study data from Medtronic at ADA
The company reported data from a real-world analysis of 3,543 children at or under 15 years old. They used MiniMed 780G with recommended settings of 100 mg/dL and two-hour active insulin time. Results from the group, based in Europe and Latin America, included 78% time-in-range.
Medtronic reported a separate analysis of the same age group (2,516 subjects) in Europe. It demonstrated improved glycemic performance with SmartGuard technology regardless of baseline glycemic control. The group with the lowest time-in-range prior to SmartGuard technology had the largest increase (23.3%).
Patients with the best metabolic control achieved 80.6% time-in-range. That increase came with less effort as evidenced by fewer user-initiated boluses, Medtronic said. This indicates decreased patient burden.
Additionally, Medtronic presented real-world data on 108 users of its seven-day Extended Wear Infusion set. The analysis showed an average infusion set wear time of 6.74 days. Nearly half of the evaluated individuals (48.2%) wore the set for seven days. The data mirrored previous U.S. results and demonstrates a reduced user burden through less frequent infusion set changes, Medtronic said.
“We’re committed to pushing simplification of diabetes management as far as we can and are heartened to see the impact our MiniMed 780G system is having on both clinical and quality of life outcomes as evidenced by our randomized controlled ADAPT study and the growing body of real-world evidence from around the world,” said Dallara. “With each advancement, we’re working to reduce more of the burden that this disease demands and will continue to innovate to make life easier for those we have the privilege to support.”
Strong year continues for Medtronic’s Diabetes business
Medtronic’s Diabetes unit has had an eventful few months.
Shortly after receiving the MiniMed approval, the company confirmed a full resolution for an FDA warning letter. In December 2021, the FDA had issued a warning letter to Medtronic’s Northridge, California, Diabetes headquarters. Medtronic said it took proactive actions to continue to strengthen its quality systems.
Not long after resolving the letter, Medtronic received another regulatory boost in Canada. The country’s regulatory body licensed the use of the next-generation Guardian 4 CGM sensor with MiniMed 780G. Canadian officials previously approved the MiniMed 780G pump in November 2022. Medtronic plans to launch the system there by the end of 2023, having begun shipments in the U.S. already.
Last month, Medtronic entered into a set of agreements to acquire insulin delivery technology developer EOFlow. Seongnam, South Korea-based EOFlow develops the EOPatch — a tubeless, wearable and fully disposable insulin delivery device.
EOFlow already launched its EOPatch insulin delivery system in Korea and Europe. The company submitted the insulin delivery device for U.S. FDA clearance in January. EOFlow already has FDA breakthrough device designation on a wearable, integrated artificial pancreas. The disposable device features a glucose monitoring sensor, insulin pump and automated insulin delivery algorithm.

