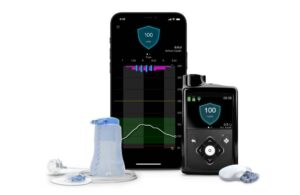
For years, clinical data backed up the MiniMed 780G insulin pump with Guardian 4 sensor technology. Over the last year alone, multiple studies further supported the platform’s impact on glycemic control and diabetes management outcomes.
But, the company hit an initial hiccup with an FDA warning letter related to its California Diabetes facility in 2021. CEO Geoff Martha said early in 2022 that the letter was likely to impact the regulatory timelines for next-generation technology. Meanwhile, analysts were suggesting that the unit was on the spinoff block.
For now, those issues are in the past, though. Medtronic yesterday said it fully resolved the warning letter with the FDA.
Just a few days earlier, Medtronic announced that it received FDA approval for MiniMed 780G with Guardian 4.
The system offers meal detection technology and provides automatic adjustments and corrections to sugar levels every five minutes. This occurs for both basal (background) and bolus (mealtime) insulin needs.
MiniMed 780G with Guardian 4 offers insulin to account for when users forget to bolus or underestimate the number of carbs in their meal. It also requires no fingersticks when in SmartGuard mode.
Analysts reacted positively to the news, as Wells Fargo and Barclays upgraded MDT stock. BTIG analyst Ryan Zimmerman isn’t jumping to conclusions too quickly on the impact on Medtronic’s business.
“We think the bigger question is whether this approval provides MDT’s Diabetes business unit with a much needed growth boost and can it lift the entire company closer to MDT’s long-term growth guidance?” he wrote.
Que Dallara, Medtronic Diabetes EVP and president, and Ali Dianaty, VP of product innovation, spoke with Drug Delivery Business News following the landmark regulatory nod.
“We’re thrilled to have 780G approved in the U.S.,” Dallara told DDBN. “At long last and much-anticipated.”
The features that set MiniMed 780G apart
Dallara called MiniMed 780G the world’s first and only system with meal detection technology. Its responsive algorithm enables the five-minute adjustments for both basal and bolus insulin needs.
“That’s a very big deal,” Dallara explained. “If you think about the original concept of the artificial pancreas, it’s the closest you can get to this dynamic closed-loop environment.”
According to Dallara, the buzz in the diabetes space has often centered around a lack of fingersticks. She said the rise of CGM “is absolutely a good thing,” but, she noted, “there’s so much more in diabetes management.”
Offering dynamic dosing to target every five minutes, correcting doses when users miss carb-counting numbers and operating throughout the night, sets the MiniMed 780G apart.
The Guardian 4 sensor requires zero calibration, according to Medtronic’s Diabetes unit head. Additionally, the seven-day infusion set enables for a set change every week, rather than three days. Dianaty said the company’s intent is for users to “every Saturday morning, change out their infusion set and change out their sensor.” They can add the insulin they need and be on their way without much left to do to maintain the system, he explained.
“All of these things together is a monumental improvement on the existing Medtronic systems on the market today,” Dallara said.
What’s different from the previous diabetes offerings from Medtronic?
Dianaty said the main underlying capability that brings MiniMed 780G forward from previous Medtronic versions is the ability to correct for highs in glucose values.
People with diabetes tend to struggle with carb counting, he explained. Diabetics can often underbid the amount of carbohydrates they’ve eaten, enabling them to do a secondary check or correction later. This occurs because of a lingering fear of dosing too much insulin, Dianaty said.
“[MiniMed 780G] automatically corrects their insulin dosing every five minutes,” Dianaty explained. “It’s able to provide these larger correction boluses more readily and more aggressively than anything else on the market.”
Another feature Dianaty stressed is the target capabilities. He compared it to a thermostat in the home — a user sets it to a specific number and wants to hit that number. Competitive devices, he said, offer a range.
“Instead of it being 72 degrees, they set it to 67 to 75 and hope for the best in between, whereas we set it at 72 and hit that mark,” he declared.
Medtronic used to set its target at 120 mg/dL, but with MiniMed 780G, the target can go down to 110 mg/dL or even as low as 100 mg/dL. Dianaty said that mirrors a healthy pancreas, where most people sit between 90 mg/dL and 100 mg/dL at baseline.
The seven-day infusion set is another new feature, while Dianaty said the company improved the algorithm for MiniMed 780G. With this improvement, 95% of the time, users remain in automation with their pump.
“That’s just peace of mind, less alarms, less alerts, less things for them to have to do in order to get the outcome that they’re looking for,” he said. “So, it’s quite a change for us as a result of these improvements in the 780G.”
What’s next?
First and foremost, Medtronic aims to transition users from previous-generation devices to the MiniMed 780G. Fortunately, for MiniMed 770G users, it’s just a software upgrade away.
Medtronic plans to begin taking pre-orders on May 15, 2023. It expects the first shipments to come in the summer. Like with any upgrade, it may take some getting used to. However, Dianaty and Dallara don’t expect much hesitancy from customers.
Dallara explained that the most diligent people managing their diabetes like to be in control. But, after a week or so, they “realize how much their system is doing for them.”
“The thing we’re reminding them of is that they can get better outcomes with less work,” Dianaty said. “As a result of that, they’re actually quite excited to get it and champing at the bit. Our phones have been ringing off the hook to get the upgrade right away.”
Still, Medtronic still feels there’s “plenty to do,” Dianaty said. That includes the challenge of overcoming carb counting altogether and predicting when meals happen to handle all aspects of diabetes.
For now, the company is “very excited and optimistic” about its prospects, Dallara noted. MiniMed 780G has been in the European market for about two years, so Medtronic is looking forward to getting the technology to U.S. users.
“The reception that we’ve seen since Friday’s announcement has been you know, short of nothing short of stellar,” Dallara said. “We can’t wait to get these in the hands of our patients. I think that the results will speak for themselves because [users are] going to experience the simplification in their diabetes management that they can’t get anywhere else.”

