ScanOfTheMonth.com publishes monthly collections of CT scans of exciting products and the month of July has some intriguing scans of drug delivery devices, including inhalers, pens and more.
The scans cover life-saving technologies that span the drug delivery space. Learn more about a few scans here:
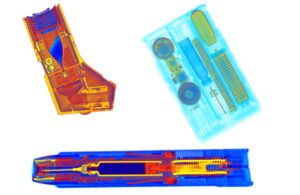
AUVI-Q
AUVI-Q (epinephrine injection, USP), developed by Kaleo, is a prescription medicine used to treat life-threatening allergic reactions, including anaphylaxis, in people who are at risk for or have a history of serious allergic reactions.
The auto-injector can be carried easily and used quickly in the case of an emergency. FDA approved the device designed to treat life-threatening allergic reactions for infants and small children in 2017.
Kaleo’s 0.1 mg Auvi-Q auto-injector has a shorter needle and a lower dose of epinephrine compared to the company’s 0.15 mg and 0.3 mg devices. The injector includes a voice prompt system that gives the user step-by-step instructions, as well as a needle that automatically retracts after administration.
Mylan EpiPen
Mylan’s EpiPen 0.3 mg epinephrine injection also is used to treat life-threatening allergic reactions, including anaphylaxis.
The device is injected into the middle of the user’s outer thigh (upper leg), through clothing if necessary.
Back in 2016, Mylan held an estimated 94% market share in the U.S., although alternative devices, such as one from Teva, have threatened the product’s dominance. The company has also faced its share of legal issues, although one of the more recent outcomes for the company was a positive one, as the U.S. District Court for the District of Kansas ruled in favor of the company in an EpiPen class action.
Mylan received a favorable ruling in substantial part that dismissed all of the plaintiffs’ claims under the federal RICO statute, including claims asserted against former Mylan CEO Heather Bresch. The court also dismissed claims alleging that Mylan foreclosed branded competition through rebate arrangements with pharmacy benefit managers.
Powder inhaler
The ProAir RespiClick is a breath-activated powder inhaler for people with asthma.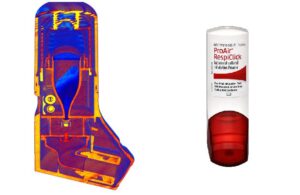
When the user is in the middle of an asthma attack, they don’t have to try and coordinate “pressing and breathing” at the same time.
Additionally, ProAir RespiClick doesn’t need to be primed or shaken, because there is no spray or “plume.” There is no need for washing and no button to press.
ProAir RespiClick (albuterol sulfate) inhalation powder is a prescription medicine indicated for use in people 4 years of age and older to treat or prevent bronchospasm in people who have reversible obstructive airway disease and/or to prevent exercise-induced bronchospasm.

