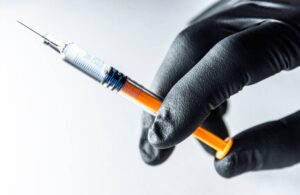
Torrington, Connecticut-based Dymax designed MD 1045-M to bond glass, SS, ABS and PC substrates. Uses for these include assembling prefilled syringes and single-use devices. They also aid in the assembly of auto, pen and wearable injectors. The company formulated MD 1045-M to solve the challenges associated with needle orientation, material overflow and long cure times.
This low-extractable material passes ISO 10993 cytotoxicity testing and meets essential biocompatibility standards for medical device applications. Dymax aims for it to help manufacturers increase productivity, reduce processing times and exceed critical pull force and quality requirements.
The one-part, solvent-free, 100% solid adhesive represents an eco-friendly alternative to solvent-based adhesives. It features low, 475 centipoise viscosity for easy flow onto components in 10 seconds. Dymax says the current standard comes in at 30 seconds or more.
It also cures tack-free with UV/broad spectrum light. MD 1045-M is LED-curable at 365 nm in five seconds or faster for environmentally friendly processing. The low-shrinkage adhesive exhibits a glass-like cure surface and resists yellowing. It withstands autoclave cleaning and Gamma and ETO sterilization methods.

