Axium Pharmaceuticals said today that the FDA accepted its orphan designation request for the intranasal administration of lorazepam, Truveta, in the treatment of Lennox-Gastaut syndrome.
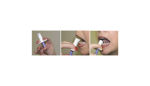
The High Point, N.C.-based company is developing a novel intranasal administration device for new formulations of existing drugs. Axium said the device is designed to bring about rapid onset of a therapeutic effect with minimal side effects, while boosting bio-availability.
The device is supposed to ease patient anxiety and produce minimal physical discomfort, according to the company. No significant drug residue is left in the intranasal delivery device after administration, Axium reported.
The company added that it is developing an array of pharmaceutical candidates for indications including neurological disorders, infectious diseases and diabetes.

