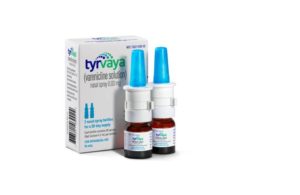
Crystal Lake, Illinois–based Aptar said the patented Cartridge Pump System (CPS) is for the multidose delivery of preserved and non-preserved drug formulations. It has received approval as the device for delivering Oyster Point Pharma’s Tyrvaya (varenicline solution) nasal spray (0.03 mg).
According to a news release, Tyrvaya delivered with Aptar’s CPS becomes the first and only nasal spray approved for treating signs and symptoms of dry eye disease in the U.S.
“This approval further demonstrates the broad potential for Aptar’s drug delivery solutions and the continued market opportunities for nasal drug delivery,” Aptar President & CEO Stephan B. Tanda said in the release.
Approval for Tyrvaya with the CPS included a combination product submission by Oyster Point to the FDA, which benefitted from Aptar Pharma’s portfolio of stage-specific development packages, the company said. Aptar’s regulatory affairs professionals and analytical scientists helped customers proactively address regulatory needs to accelerate approval, too.
“We are pleased that Aptar Pharma’s CPS Technology has been successfully reviewed by the U.S. FDA for Oyster Point Pharma’s therapy for the treatment of the signs and symptoms of dry eye disease via the nose,” Aptar Pharma President Gael Touya said. “This project once again underlines Aptar Pharma’s ability to develop and deliver complex drug delivery systems, and demonstrates our expertise in partnering with customers through their successful drug development journeys.”

