 The FDA issued a safety communication last week detailing the difference between standard pen needles and safety pen needles, both of which are used to inject medicine via pen injectors.
The FDA issued a safety communication last week detailing the difference between standard pen needles and safety pen needles, both of which are used to inject medicine via pen injectors.
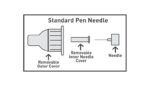
A standard pen needle usually has an outer cover and a removable inner needle cover – both covers must be removed prior to an injection. A safety pen needle has an outer cover and a fixed inner needle shield. While the outer cover is removed before an injection, the fixed inner needle shield remains in place.
It’s easy to confuse the two types of needles, the FDA noted, and sometimes patients are trained using one type of pen needle rather than the other. Both pen needles can be used with pen injectors.
The U.S. regulatory agency said it has received reports of patients using standard pen needles to inject insulin without removing the inner needle cover, preventing the needle from entering the skin and delivering the insulin. The FDA knows of at least one patient who was hospitalized and later died due to hyperglycemia after incorrectly injecting their insulin.
To ensure the safe use of pen needles, the FDA recommended that patients check each new box of pen needles. The agency also said that patients should consult their healthcare provider if they have any concerns that their pen injector is not working.
The FDA has asked pen needle manufacturers to review their most recent labeling and training materials to evaluate the need for any potential updates. Standard pen needle makers should also consider adding a warning to their products’ labeling, noting that users need to remove both the outer and inner needle cover, according to the FDA.

