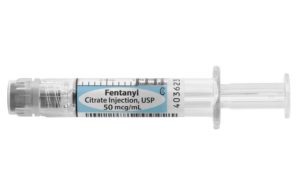
Lake Zurich, Ill.-based Fresenius Kabi’s proprietary Simplist ready-to-administer prefilled syringe offers the fentanyl citrate injection at 50 mcg per 1 mL in an effort to reduce waste and diversion while ensuring the safe delivery of medication.
In a news release, the company touted the fentanyl citrate injection in the Simplist syringe as the only 1 mL presentation available in the U.S. today.
Simplist comes in Fresenius Kabi’s proprietary MicroVault packaging system designed to provide secure dispensing of narcotics and reduce the opportunity for diversion. The company said the Simplist prefilled syringes are associated with an error rate that registers four times lower than traditional medication administration practice.
The Simplist fentanyl single-unit dose prefilled syringe offers a 24-month shelf life. Its indications include:
- Analgesic action of short duration during the anesthetic periods, premedication, induction and maintenance and in the immediate postoperative period (recovery room) as the need arises.
- Use as an opioid analgesic supplement in general or regional anesthesia.
- Administration with a neuroleptic as an anesthetic premedication, for the induction of anesthesia and as an adjunct in the maintenance of general and regional anesthesia.
- Use as an anesthetic agent with oxygen in selected high-risk patients, such as those undergoing open heart surgery or certain complicated neurological or orthopedic procedures.
“The introduction of an exclusive smaller-dose fentanyl injection is an important expansion of our Simplist ready-to-administer prefilled syringe portfolio in line with our commitment to the secure dispensing of controlled substances for the safe delivery of drugs to patients,” Fresenius Kabi USA president & CEO John Ducker said in the release. “We are proud to design, develop and introduce products and labeling that support the safe dispensing of controlled substances, and all products.”

