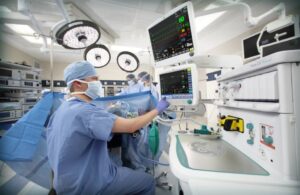
Approval covers the use of the Et control software for general anesthesia delivery on GE Healthcare’s Aisys CS2 anesthesia delivery system. The technology was released in Europe in 2010 and today can be used to deliver anesthesia in more than 100 countries.
The Et control software semi-automates anesthesia delivery to allow providers to set targets for end-tidal oxygen and anesthetic agent, according to a news release. Once the targets are set, the software achieves and maintains the targets regardless of changes in a patient’s hemodynamic and metabolic status, improving anesthesia delivery accuracy and simplifying workflows while reducing drug waste, lowering the cost of care and lowering greenhouse gas emissions, GE Healthcare said.
Et control software may allow for a potential 44% decline in greenhouse gas emissions as a result of more efficient use of anesthetic agents, plus increased accuracy, a 50% reduction in manual keystrokes and a potential 27% reduction in operating room costs.
“Anesthesia providers in the U.S. will have access to the most advanced anesthesia tools available to improve patient care,” Eric Ruedinger, GM of GE Healthcare’s Anesthesia and Respiratory Care business, said in the release. “As the long-standing global leader in anesthesia delivery, GE Healthcare invested in the development and clinical validation of this Et Control algorithm, and we are committed to creating clinically relevant solutions that will enhance anesthesia practices into the future.”

