The money will mostly pay for a Phase 1 clinical study in which Vaxxas’ high-density micro-array patch (HD-MAP) delivering pandemic influenza vaccine to more than 400 people. Researchers chose pandemic influenza vaccine for the $24.1 million study in order to comprehensively baseline the immune responses and safety of the novel HD-MAP vaccination platform.
Vaxxas (Cambridge, Mass.; Brisbane, Australia) is also actively investigating opportunities to improve the performance of other pandemic vaccines including against COVID-19, as well as a broad range of non-pandemic infectious disease vaccines.
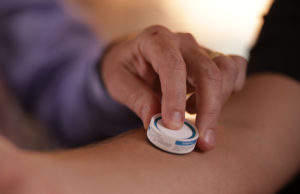
Based on technology originally developed at the University of Queensland, Vaxxas’ HD-MAP includes a 9-by-9 mm array of thousands of very short projections around 250 microns in length. Invisible to the naked eye and coated with vaccine, the projections can quickly deliver vaccine to immune cells. Vaxxas officials claim the technology can deliver vaccine more efficiently than a needle and syringe.
“We are excited to partner with BARDA to rapidly deploy Vaxxas’ HD-MAP technology, which holds the promise of significantly improving pandemic response with needle-free vaccine delivery that is more effective and readily accepted,” Vaxxas CEO David L. Hoey said in a news release.
“Having validated our HD-MAP technology in clinical studies at commercial scale, we stand ready to be among the leading innovators who can solve critical challenges to global pandemic health crises, going beyond and enhancing the capabilities enabled by promising vaccines,” Hoey said.
Vaxxas collaborators include Merck (NYSE:MRK), the World Health Organization and the Bill and Melinda Gates Foundation. German manufacturing equipment maker Harro Höfliger has agreed to help Vaxxas develop a high-throughput, aseptic manufacturing line.

