 Voluntis said today that its digital management system for people with Type II diabetes, Insulia, won FDA clearance and CE Mark approval to integrate basal insulin brands Tresiba and Basaglar. The company’s software already works with Lantus, Toujeo and Levemir.
Voluntis said today that its digital management system for people with Type II diabetes, Insulia, won FDA clearance and CE Mark approval to integrate basal insulin brands Tresiba and Basaglar. The company’s software already works with Lantus, Toujeo and Levemir.
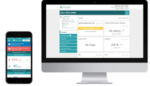
The Insulia digital companion is composed of a patient mobile app and healthcare worker web portal. The app gives real-time basal insulin dosing recommendations and educational coaching messages based on blood glucose levels to patients with Type II diabetes.
The web portal enables healthcare practitioners to develop personalized treatment plans and track their patient population remotely, Voluntis touted.
The company is working on an extension for the app that would include other insulin therapies, like GLP-1 basal insulin combos, and is aiming for a release in 2018, according to Voluntis.
“It’s complicated enough worrying about how to titrate your insulin, without having to worry about whether your brand of insulin is compatible with automatic titration tools such as Insulia,” CEO Pierre Leurent said in prepared remarks. “We are very excited to make Insulia available to all those with Type II diabetes, regardless of the basal insulin brand they’re using.”

