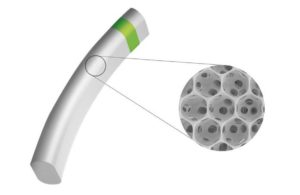
MINIject, a minimally invasive glaucoma surgery (MIGS) device, treats open-angle glaucoma. Currently the only commercially available supraciliary MIGS implant, it has demonstrated meaningful and sustained performance, plus a favorable safety profile.
The device combines the porous structure of iStar’s proprietary STAR material with the power offered by the supraciliary space to enhance natural fluid outflow, reduce intraocular pressure and the need for medication while bio-integrating with surrounding tissue, limiting inflammation, fibrosis and subsequent complications.
It holds CE mark approval, and garnered FDA investigational device exemption (IDE) in July 2021.
Wavre, Belgium-based iSTAR said Dr. Anish Dhital successfully implanted MINIject in patients at the Institute of Eye Surgery, Mullingar. The addition of Ireland to the company’s commercial rollout follows expansion into other European countries, including Sweden and Norway.
Dhital said the implant can “significantly and safely lower [intraocular pressure].” He added that it targets a new drainage pathway, benefitting patients and enabling them to reduce their reliance on medication. Dhital noted that it could also help to preserve vision for longer.
“It is both exciting and validating that one of Ireland’s largest network of eye clinics to offer all ocular sub-specialties, the IOES, has engaged with our technology and we want to thank the clinicians for their commitment,” said iSTAR CEO Michel Vanbrabant. “The continuation of our commercial rollout of MINIject with the recent implantations in Ireland represents iSTAR Medical’s mission to preserve the vision of glaucoma patients around the world. We are proud to be providing more patients with a safe and minimally-invasive treatment for open-angle glaucoma, giving them a sustained and prolonged treatment option. As we continue with our global expansion, we look forward to making MINIject more widely available to patients.”

