 Researchers from the University of Washington developed synthetic nanocarriers to encase chemotherapy drugs and deliver them directly to the tumor site, avoiding healthy tissue. The team’s work was published in the journal Small.
Researchers from the University of Washington developed synthetic nanocarriers to encase chemotherapy drugs and deliver them directly to the tumor site, avoiding healthy tissue. The team’s work was published in the journal Small.
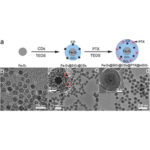
The nanocarrier is a hybrid of synthetic materials designed to allow the system to carry drugs and fluorescent particles to stain the tumor for imaging by surgeons.
“Our nanocarrier system is really a hybrid addressing 2 needs – drug delivery and tumor imaging,” senior author Miqin Zhang said in prepared remarks. “First, this nanocarrier can deliver chemotherapy drugs and release them in the tumor area, which spares healthy tissue from toxic side effects. Second, we load the nanocarrier with materials to help doctors visualize the tumor, either using a microscope or by MRI scan.”
In previous attempts to develop nanocarriers, researchers would develop an empty, synthetic carrier and then try to encapsulate the therapeutic drug. But this came with a high risk of damaging the drugs and rendering them ineffective.
“Most chemotherapy drugs have complex structures – essentially, they’re very fragile – and they do no good if they are broken by the time they reach the tumor,” Zhang explained.
Instead, Zhang’s team developed a “load during assembly” technique, in which the nanocarrier is assembled and loaded with chemotherapy drugs simultaneously.
The nanocarrier has an iron oxide core, surrounded by a shell of silica. The silica-based shell was designed specifically to stack the chemotherapy drug paclitaxel. The team also devised the nanocarrier to have space for carbon dots – small particles used to image tissue under a microscope.
Zhang said the nanocarrier, which when fully loaded is less than the thickness of a sheet of notebook paper, can provide imaging for months.
The team of researchers tested the nanocarriers by injecting healthy mice with empty nanocarriers and loaded nanocarriers. Checking the vital organs 5 days following injection, the team saw no evidence of toxicity.
“This would indicate that the nanocarriers themselves do not trigger an adverse reaction in the body, and that the loaded nanocarriers are keeping their toxic cargo shielded from the body,” Zhang said.
Once the nanocarriers reach a tumor, gentle heating from a low-level infrared light breaks apart the carriers and releases the chemotherapy drugs. The team tested this by injecting empty and loaded carriers into mice with cancerous tumors. Mice injected with empty nanocarriers showed no reduction in tumor size, but the tumors in mice injected with loaded carriers shrank significantly. The team saw similar results with human cancer cells cultured and tested in the laboratory.
“These results show that the nanocarriers can deliver their cargo intact to the tumor site,” Zhang said. “And while we designed this nanocarrier specifically to accommodate paclitaxel, it is possible to adjust this technique for other drugs.”

