Medtronic’s fiscal years wrap up at the end of April.
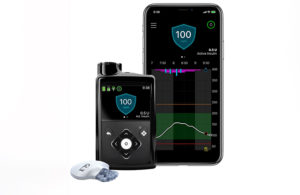
Speaking on the company’s second-quarter earnings call, Chair & CEO Geoff Martha said Medtronic expects its MiniMed 780G insulin pump system with the Guardian 4 sensor for continuous glucose monitoring (CGM) to drive growth once approved, with the company currently in talks with the FDA.
“We’ve got a really strong uptake of 780G and Guardian 4 outside the U.S. and inside the U.S., we’re waiting for that approval to come through,” Medtronic EVP & President of its Diabetes Operating Unit and Cardiovascular Portfolio Sean Salmon said on the call. “We’re making progress with the FDA.”
Previous generations of MiniMed insulin pumps have come under scrutiny. Still, Medtronic as recently as last month touted one-year clinical data for the next-generation MiniMed 780G with Guardian 3 in children. Company officials expect Guardian 4 to deliver positive outcomes as well.
Additionally, Martha touched on the Simplera disposable CGM sensor (formerly Synergy), which he touted as half the size of the Guardian 4 sensor. The company expects to bring the Simplera sensor to the FDA later this fiscal year.
Finally, when asked by an analyst if Medtronic had any plans to spinoff its diabetes business, Martha said there are no current plans, but the executive committee at the medtech giant is spending “a lot more time” looking at portfolios and capital allocation to “make sure we’re the right owner.”
“I do see opportunities,” Martha said. “I’m not signaling anything, but I can tell you this is something we’re constantly looking at. I don’t see ourselves as [General Electric] or [Johnson & Johnson] with dramatically different businesses, but I do believe it is an opportunity over time.”

