That’s according to the Diabetes Patient Voice survey (DQ&A) — a third-party survey that evaluates U.S. diabetes patient satisfaction on a quarterly basis.
However, things are now looking up for the world’s largest medtech company in the diabetes tech space.
The old third-place ranking was for the previous-generation MiniMed 670G — before the company brought its next-generation MiniMed 780G system to the market.
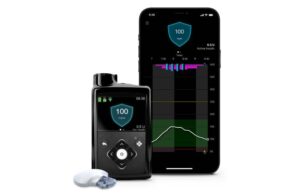
The current iteration of MiniMed 780G uses the latest Guardian 4 technology and requires no fingersticks while in “SmartGuard” mode. It provides meal detection technology providing automatic adjustments and corrections to sugar levels every five minutes. This occurs for both basal (background) and bolus (mealtime) insulin needs.
MiniMed 780G with Guardian 4 offers insulin to account for when users forget to bolus or underestimate the number of carbs in their meal.
In the latest DQ&A survey of more than 6,000 patients with both type 1 and type 2 diabetes, the fourth-quarter 2024 survey, MiniMed 780G ranked first in patient satisfaction.
The system scored highest in satisfaction (64%) compared to competing pumps. That includes the Tandem t:slim X2/Dexcom G6 (57%) and Omnipod 5/G6 (44%). Results showed that satisfaction with the Guardian 4 sensor mirrors that of Dexcom and Abbott’s sensors, too. That came in at an all-time high following the introduction of MiniMed 780G.
“We’ve always been known for having strong outcomes, but we also had more of a burden profile,” Medtronic Diabetes VP, Global Marketing & Communications, Krista Sugerman told Drug Delivery Business News in a recent interview. “It just wasn’t quite as easy to use our systems. We’ve really been working toward that and we’re excited about our pipeline and being able to make it easier for people living with type 1 diabetes.”
Medtronic raises the bar on outcomes
According to Sugerman, Medtronic sees the biggest difference with its latest pump system comes with its automation capabilities. Auto corrections and carb counting ease the burden of managing diabetes, with algorithms doing the work for patients.
“That was a big focus for us,” Sugerman said. “We knew that what we wanted to was raise the bar on outcomes. We don’t want to jeopardize time in range and patient outcomes. But, we knew we had to make it easier, more customer-centric, more patient-centric. And that’s really what we’ve done.”
One point of interest there was alerts and alarms, as Medtronic set out to reduce them altogether. Sugerman said the survey reflected that effort, with patients ranking MiniMed 780G highest in terms of alarms and alerts.
“That was a dramatic difference from our previous system,” she explained. “They’re able to see outcomes and achieve the highest time in range but with less burden, less alarms and alerts, less distractions from their day-to-day lives. The meal detection technology and the auto corrections are taking care of that for them.”
Sugerman highlighted a meeting she had with a customer in which the customer told her that using MiniMed 780G was “the closest I’ve come to feeling like I have a normal functioning pancreas.”
That’s not an isolated outcome, either, she said.
“I don’t want to oversell or over-promise, but when the patients are telling us that, it’s really rewarding,” Sugerman said. “It’s great to hear.”
‘The ability to expand’
To continue to “raise the bar” and reach more people with the MiniMed technology, Sugerman sees the opportunity to expand. Historically, she says, doctors reserved pump therapy for people who must meet a certain threshold. They often need to be good carb counters, tech-savvy and more.
But, with AI-automated insulin delivery, Medtronic can drive results for more users, she said. One target area is patients who start on CGM alone. According to Sugerman, less than 30% of patients on CGM alone achieve their glycemic targets.
“What we want to really do by raising outcomes, lowering the burden and expanding access, is making sure that more of a wide range of people have access to insulin therapy,” she said. “That’s a big part of what we’re doing with [MiniMed] 780[G].”
Part of that expansion of access includes giving more patients a choice with their MiniMed 780G. In addition to the combination with Guardian 4, Medtronic has a newer version on the way. Its system garnered CE mark with the next-generation Simplera Sync sensor in January.
Sugerman said Simplera, which is under review with the FDA for U.S. marketing authorization, can help catch the company up to the rest of the competition on the CGM side.
With the capabilities of MiniMed 780G, the company believes it can move the focus to outcomes, time in range or time in tight range, taking it away from sensor choice to more of a system and algorithm choice within automated insulin delivery.
“Now that sensors are starting to catch up… a lot of the feedback we’re hearing is that sensors will become a commodity over time,” she said. “So, the algorithms and the outcomes are going to be the differentiating factors.”

