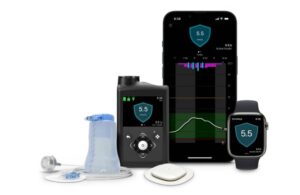
Simplera Sync, a disposable, all-in-one continuous glucose monitor (CGM), eliminates the need for fingersticks and overtape. It features an improved user experience at half the size of previous Medtronic sensors with a simple, two-step insertion process.
MiniMed 780G, an automated insulin delivery system, offers meal detection technology and provides automatic adjustments and corrections to sugar levels every five minutes. This occurs for both basal (background) and bolus (mealtime) insulin needs.
The pump, which also works with Medtronic’s Guardian 4 sensor, offers insulin to account for when users forget to bolus or underestimate the number of carbs in their meal. It also requires no fingersticks when in SmartGuard mode. With the world’s only 7-day infusion set, MiniMed 780G offers low glucose target settings (as low as 100 mg/dL).
Simplera, Medtronic’s next-generation CGM, also works with the InPen device. It launched with InPen in Europe last year after earning CE mark. Simplera Sync, which leverages Medtronic’s automated insulin delivery algorithm, has a similar look and feel to the Simplera device.
Medtronic plans to make MiniMed 780G with Simplera Sync available in Europe through a limited release in spring 2024. It then expects a phased commercial launch in the summer of 2024. The system’s current indication covers ages seven and up with compatibility with iOS and Android devices. It remains investigational in the U.S.
“We’re incredibly proud that the MiniMed 780G system continues to be the most widely used automated insulin delivery system in Europe since we launched it in 2020,” said Que Dallara, EVP and president, Medtronic Diabetes. “With the introduction of Simplera Sync sensor, we’re able to offer the proven benefits of our MiniMed 780G system with our newest and most comfortable sensor that can be applied in under 10 seconds.”
