 As a dentist, Mark Hochman gave countless injections to his patients and watched as many of them would cringe from pain. Frustrated with a technology that he said hasn’t improved in decades, he decided to take a different approach to the common method of drug delivery.
As a dentist, Mark Hochman gave countless injections to his patients and watched as many of them would cringe from pain. Frustrated with a technology that he said hasn’t improved in decades, he decided to take a different approach to the common method of drug delivery.
“A team of us got together to evaluate whether we could change the way that subcutaneous drug delivery was being performed in this country and around the world,” Hochman told Drug Delivery Business News. “We realized that as one of the many routes of administration for drug delivery, it was being practiced on a device that was over 160 years old, which is basically the conventional syringe.”
Twenty years later, he now serves as director of clinical affairs and R&D at Milestone Scientific (NYSE:MLSS), working to bring the company’s CompuFlo system to patients and healthcare providers.
Milestone Scientific has developed a device that incorporates Hochman’s dynamic pressure sensing technology to precisely control the flow rate of fluid during an injection. The system uses real-time feedback based on pressure, which is measured at the tip of the needle, to control where and how quickly a drug is released into the body.
Identifying where a needle is in the body during a traditional subcutaneous injection is a subjective process, according to Hochman.
“What we’ve done is we’ve brought that into the 21st century,” he said. “We use an electromechanical motor that is very, very precise in its movements.”
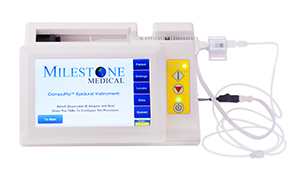 The CompuFlo system, which consists of a motor, a touch-screen user interface and a disposable syringe, can administer subcutaneous injections in a “virtually painless” manner, according to the company.
The CompuFlo system, which consists of a motor, a touch-screen user interface and a disposable syringe, can administer subcutaneous injections in a “virtually painless” manner, according to the company.
After the company launched its first product, the Wand STA system, for use in the dental space, the team learned that they could differentiate between tissue types based on pressure signatures, enabling healthcare practitioners to target drugs to specific tissues.
Milestone Scientific has expanded from dentistry to include technologies designed for intra-articular injections and, most recently, an instrument for epidural injections. Both CompuFlo devices have CE clearance in the European Union and last month, its epidural product won 510(k) clearance from the FDA.
Hochman said that in the past, researchers have focused on reformulating drugs to address the issues involved with subcutaneous drug delivery. Instead, he decided to take aim at upgrading and redesigning the device itself.
“The conventional syringe was really initially designed primarily for convenience,” he said. “It was never designed for sophisticated fluid dynamics. It was never designed with the primary objective of changing pain perception or trying to do anything beyond pushing a drug into tissues.”
Looking ahead, Milestone Scientific is finishing up development of a cosmetic instrument for the delivery of drugs like Botox. Hochman also said it is looking to apply its CompuFlo system for peripheral nerve blocks, as a means of delivering regional anesthesia.
“We have some initial information demonstrating that this is an area that the technology can be used quite effectively,” he said.
But Hochman added that the company is positioning its pressure-sensing, drug-delivery technology as a system that could have an array of potential applications.
“We’re looking towards a platform of different technologies based on dynamic pressure sensing,” he said. “We’re really excited about what the future holds, because it’s showing that it can benefit people in ways that we haven’t thought of and that it makes [subcutaneous injections] more predictable for the practitioner, as well. There’s really no reason why we should be subjected to these injections using a technology that’s over 150 years old.”

