 Researchers from North Carolina State University and the University of North Carolina at Chapel Hill have designed a glucose-responsive “smart” insulin delivery system using modified insulin and red blood cells.
Researchers from North Carolina State University and the University of North Carolina at Chapel Hill have designed a glucose-responsive “smart” insulin delivery system using modified insulin and red blood cells.
In an animal study of mice with Type I diabetes, the system effectively reduced blood sugar levels for 48 hours. The team’s work was published in Advanced Materials.
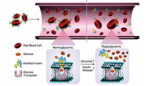
The team chemically bound insulin to a glucose derivative known as glucosamine, which in turn bound to glucose transporters on the surface of a red blood cell. The final product is a red blood cell studded with insulin molecules, the researchers reported.
Once the insulin-ferrying blood cells were inside the bloodstream, they interacted with their surroundings. If blood sugar levels are high, glucose molecules displaced the glucosamine in the blood cells’ glucose transporters. When the glucosamine is released from the blood cell, the insulin went with it. This leaves the insulin free to bind to receptors in the liver, muscles and fatty tissues, initiating a process that lessens blood sugar levels.
“In short, this is a fully biocompatible smart system that responds, when needed, to normalize glucose levels in the blood,” co-corresponding author Zhen Gu said in prepared remarks.
The team compared mice receiving the smart insulin delivery system with a group of mice that received saline solution, a group that received just modified insulin and a group that got a mixture of unmodified insulin and red blood cells.
They reported that the diabetic mice that received the smart system were able to signficantly reduce blood sugar levels for more than 48 hours. On the other hand, the best performance the team saw among the other groups was an initial dip in blood sugar that returned to high glucose levels within 12 hours.
The team also evaluated each of the drug combinations in a group of healthy mice and saw that the smart delivery system lessened the risk of hypoglycemia.
Finally, the team conducted an experiment using modified insulin and nanoparticles coated with red blood cell membranes. This system demonstrated comparable results to the modified insulin and red blood cell system.
“This is a positive result, because it bodes well for developing a standardized means of delivering this glucose regulation system,” Gu said.
“The team will further evaluate the long-term biocompatibility of the modified insulin system in an animal model before determining whether to move to clinical trials,” co-author Dr. John Buse added. “The vision, if realized, would be one of the most exciting advances in diabetes care.”
“We are also exploring the use of painless microneedles to deliver this system, rather than relying on the conventional injections which were used in this study,” Gu said. “The possibility of using a different drug delivery system makes it difficult to assess cost, but we’re optimistic that the cost of the potential formulation would be comparable to existing treatments.”

