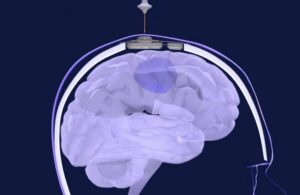
The device opened the blood-brain barrier to repeatedly permeate large, critical regions of the human brain. This enabled the delivery of chemotherapy injected intravenously.
With the patient awake, a four-minute procedure opens the blood-brain barrier and patients go home after a few hours. Results from the Northwestern study demonstrated both a safe and well-tolerated treatment. Some patients even reached up to six cycles of chemotherapy treatment.
The paper published on May 2 in The Lancet Oncology.
Get the full story at our sister site, Medical Design & Outsourcing.

