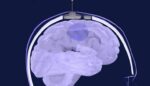
Carthera announced today that it enrolled the first patient in the SONOBIRD pivotal trial for its SonoCloud device. Paris, France-based Carthera designed SonoCloud to treat a wide range of brain disorders. The device emits ultrasound to temporarily increase the permeability of blood vessels in the brain, improving therapeutic molecule delivery. After implantation in the skull, […]
Neurological
NeuroOne is pursuing drug delivery with neural probes
NeuroOne Medical Technologies (Nasdaq:NMTC) announced today that it received a patent for drug delivery with neural probe devices. The United States Patent and Trademark Office (USPTO) issued the patent titled “Agent-Delivering Neural Probe Devices and Related Systems and Methods.” It covers novel electrodes that can operate as a standard neural electrode that delivers a treatment […]
Better Therapeutics wins FDA nod for type 2 diabetes digital therapeutic
Better Therapeutics (Nasdaq:BTTX) announced that the FDA authorized its AspyreRx prescription digital therapeutic for diabetes. AspyreRx, formerly BT-001, provides cognitive behavioral therapy to patients 18 years or older with type 2 diabetes. The prescription-only digital therapeutic (PDT) underwent review through the FDA de novo pathway. The company submitted an FDA de novo request in September 2022 for […]
ClearPoint Neuro enters drug delivery licensing deal with UCB
ClearPoint Neuro (Nasdaq:CLPT) announced today that it entered into a multi-year license agreement with UCB over gene therapy delivery. Solana Beach, California-based ClearPoint Neuro develops a therapy-enabling platform providing navigation and delivery to the brain. The licensing agreement with UCB establishes a partnership on delivery platforms for UCB’s gene therapy portfolio. “We are extremely excited […]
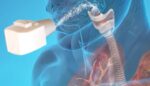
Researchers use skull-implantable ultrasound to help deliver chemotherapy to the brain
Northwestern Medicine shared results from a first-in-human clinical trial for a skull-implantable ultrasound device that supports chemotherapy delivery. The device opened the blood-brain barrier to repeatedly permeate large, critical regions of the human brain. This enabled the delivery of chemotherapy injected intravenously. With the patient awake, a four-minute procedure opens the blood-brain barrier and patients […]
Akili lays off 46 workers
Akili announced that its board of directors approved an operating plan for 2023 that includes a 30% workforce reduction. The Boston-based digital therapeutic developer’s reduction reaches across different areas and functions. In total, 30% of Akili’s workforce amounts to 46 employees. The company communicated the decision to employees on Jan. 12 and expects to complete […]
Bionaut Labs closes $43M Series B for robotic drug delivery tech
Robotic drug delivery technology developer Bionaut Labs announced today that it closed a Series B financing round worth $43.2 million. Los Angeles-based Bionaut Labs uses microscale robots to deliver drugs for treating central nervous system (CNS) diseases and disorders. Through magnetic propulsion, the company’s Bionauts can navigate the human body and deliver drugs locally. The […]
Corium launches transdermal patch for treating Alzheimer’s
Corium (Nasdaq:CORI) today announced the availability of Adlarity (donepezil transdermal system) for prescription use in the U.S. Adlarity, a once-weekly patch, delivers consistent doses of donepezil through the skin. It treats patients with mild, moderate, or severe dementia of the Alzheimer’s type. It holds FDA approval — the first and only — for 5 mg/day […]
Pulmatrix completes patient dosing for inhaled migraine treatment
Pulmatrix (Nasdaq:PULM) announced today that all subjects completed dosing in a trial of its orally inhaled migraine treatment. Lexington, Massachusetts-based Pulmatrix is evaluating PUR3100 in a Phase 1 trial using the patented iSperse technology. All subjects completed dosing of the novel orally inhaled formulation of dihydroergotamine (DHE). The company expects Phase 1 data in the […]
FDA approves ‘first-in-market’ autoinjector for treating prolonged seizures
Rafa Laboratories announced today that it received FDA approval for its 10mg Midazolam autoinjector for treating status epilepticus, or prolonged seizures. Jerusalem-based Rafa developed the autoinjector through cooperation with the U.S. Department of Defense’s (D0D) Joint Program Executive Office for Chemical, Biological, Radiological and Nuclear Defense (JPEO-CBRND). Rafa’s Midazolam 10mg autoinjector received FDA indication for […]