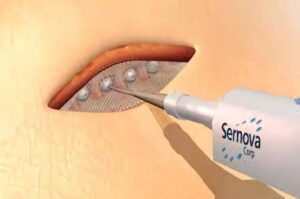
London, Ontario-based Sernova designed its proprietary Cell Pouch system as an implantable and scalable medical device that forms a natural environment in the body for long-term survival and function of therapeutic cells that release necessary proteins or factors missing from the body to treat chronic diseases, including insulin-dependent diabetes.
Data from the Phase 1/2 clinical trial, led by Dr. Piotr Witkowski at the University of Chicago, were presented at the American Diabetes Association (ADA) 82nd Scientific Sessions in New Orleans. According to a news release, the study evaluated six patients with long-standing, insulin-dependent type 1 diabetes and hypoglycemia unawareness prior to treatment.
“The data coming out of the Cell Pouch and islet transplantation trial show incredible promise for insulin-dependent diabetes patients,” Witkowski said in the release. “I am excited about the potential impact on the field and look forward to seeing continued progression of the data from this trial.”
The six patients underwent both Cell Pouch implantation and islet transplantation. Graft function was measured by blood glucose, patient insulin usage and C-peptide, a measure of islet function. The first three patients achieved complete and sustained insulin independence. The other three did not maintain optimal immunosuppression, which has now been resolved, enabling those patients to receive further protocol-defined islet transplants, Sernova said.
Cell Pouch implantation was generally well-tolerated with a favorable safety profile. All patients with favorable immunosuppression achieved complete insulin independence, with HbA1c falling in the normal range, registering at 5.0%, 5.2% and 5.2% for the three patients.
“Despite some advances in the diabetes space, insulin-dependent diabetes patients continue to require numerous injections daily, and for those with hypoglycemia unawareness, it can be increasingly life threatening. We are committed to developing a ‘functional cure’ to potentially free patients from the life-limiting burdens of this disease,” Sernova President and CEO Dr. Philip Toleikis said. “We are encouraged by the data demonstrating our Cell Pouch system has been well tolerated and multiple patients remain insulin independent. We remain on track to further dose the three aforementioned subjects and to implement the higher capacity Cell Pouch system in the next implanted patients.”

