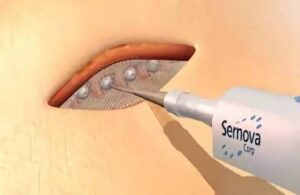
London, Ontario-based Sernova designed its proprietary Cell Pouch System as an implantable and scalable medical device. It forms a natural environment in the body for the long-term survival and function of therapeutic cells. Those cells release necessary proteins or factors missing from the body to treat chronic diseases, including insulin-dependent diabetes.
The Phase 1/2 trial evaluates type 1 diabetes patients with severe hypoglycemia unawareness. Dr. Piotr Witkowski conducts the study at The University of Chicago. Results were presented at the American Diabetes Association (ADA) 83rd Scientific Sessions.
Sernova announced positive data from that trial in June 2022. That includes three patients achieving insulin independence.
The company said in a news release that the presentation discussed the first 11 patients enrolled across two cohorts in the trial. It evaluates Cell Pouch in combination with pancreatic islets, reconfirming the safety of the system for up to more than four years following implant. During the trial, investigators measure the function of the transplanted islets by blood glucose levels, patient insulin usage and serum C-peptide.
“I continue to be encouraged by the results achieved in this trial, and especially by the ease of use and favorable response from a single islet transplant to the larger Cell Pouch,” said Witkowski. “I look forward to reporting further data later this year.”
Key points from the Sernova trial
To date, five patients in the first six-person cohort of people with completed implantation, islet transplant to Cell Pouch and supplemental portal vein islet infusion, continue to experience insulin independence for periods ranging from six months to more than three years. The sixth patient only recently completed the protocol-defined islet transplants. That subject awaits assessment of their islet graft function, Sernova said.
Additionally, Sernova reported on a second cohort with the 10-channel Cell Pouch (more than 50% greater transplant capacity compared to the previous eight-channel system). Five of seven patients meeting eligibility criteria enrolled in the second cohort and received their higher-capacity Cell Pouch implant. Three of the five received a first islet transplant to their implanted Cell Pouches. The first evaluable patient in that cohort demonstrated persistent fasting and stimulated serum C-peptide levels.
Sernova said subjects continue to tolerate long-term Cell Pouch implantation well. It demonstrated a favorable safety profile in patients receiving both types of pouches.
Five of six patients in the first cohort achieved insulin independence following supplemental islet transplants. These transplants took place via the portal vein and registered below the typical intraportal islet dose. Sernova said this indicates that islet graft function in the eight-channel Cell Pouch supports ongoing glucose control.
The company expects to report further data from the second cohort in the second half of 2023.

