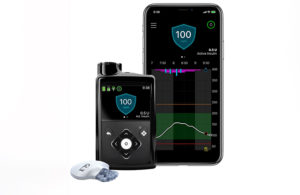
Results from the ADAPT study, published in The Lancet Diabetes & Endocrinology, demonstrated improved glycemic control and treatment satisfaction among users of MiniMed 780G.
The MiniMed 780G system is available in more than 60 countries. It is currently under FDA review. Medtronic is hopeful for clearance during the current fiscal year. If clearance takes place, it would be good news for a Diabetes business that has faced regulatory troubles. (The company recently said that it’s achieved 90% of the FDA’s action items.)
More about the ADAPT study
The study enrolled 82 individuals with type 1 diabetes not currently meeting glycemic targets. It evaluated MiniMed 780G against the standard of care (multiple daily injections plus continuous glucose monitoring). On average, enrollees scanned CGM frequently (~9 scans/day) but had suboptimal HbA1C above 8% at baseline.
Medtronic’s study randomized half of the participants to stay on standard of care, with the rest directly transitioned to MiniMed 780G. Results demonstrated improvements in glycemic targets for those on MiniMed 780G with a significant and sustained 1.4% HbA1C reduction at six months.
Users of MiniMed 780G also saw a 27.6% increase in time in range. That amounted to 6.6 more hours per day in target range compared to standard of care. Users also saw no increase in time in hypoglycemia. The improvement proved even greater overnight with the algorithm in full control.
“The ADAPT study illustrates that insulin pump therapy with advanced algorithms, like that of the MiniMed 780G system, can produce significantly improved clinical results versus the current standard of care,” said Dr. Ohad Cohen, senior global medical affairs director, Medtronic Diabetes. “Studies like this can change how health care systems define standard of care and expand options for people living with diabetes to begin using insulin pumps sooner to improve their glycemic control and help reduce the burden of diabetes.”
At six months, 27.8% of MiniMed 780G users achieved HbA1c below 7% at six months. No individuals remaining on MDI and CGM achieved that result.
Users of MiniMed 780G spent 95.8% of their time in SmartGuard advanced hybrid closed-loop delivery mode. They experienced few system exits (0.9 per week). They used the sensor 92.2% of the time compared to 87.3% in the standard of care group. The study demonstrated a significant increase in treatment satisfaction and a reduction in fear of hypoglycemia.
Medtronic said the use of MiniMed 780G — even when paired with the Guardian 3 sensor that requires two fingerstick calibrations per day — significantly improved all glycemic metrics compared to the standard of care. The company concluded that it supports use at early stages in the treatment pathway.

