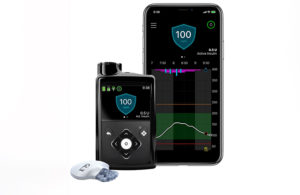
Last month, Medtronic received an FDA warning letter highlighting inadequacies in specific medical device quality system requirements at its diabetes business’ Northridge, California, facility. Today at the 40th Annual J.P. Morgan Healthcare Conference, CEO Geoff Martha said there is now some uncertainty over the timing of approval for next-generation platforms, including the MiniMed 780G insulin pump and Guardian 4 continuous glucose monitoring (CGM) sensor.
“Our extensive remediation efforts are well underway,” Martha said. “We’re working to resolve the warning letter as quickly as possible.”
Martha said Medtronic has confidence in its diabetes business, despite being unable to share much information on its mid-to-long-term pipeline because of competitive reasons. However, he said the company is currently advancing multiple programs, including what he labeled competitive CGM and patch-pump technology.
“We’re confident that we can bring value to patients and create value for shareholders,” Martha said.
Launches are expected in the coming quarters for the MiniMed 780G pump and Guardian 4 sensor. Medtronic’s portfolio also includes the Simplera disposable CGM sensor (formerly Synergy), among other undisclosed development programs.
Martha confirmed that the company’s investment in medtech over the mid-to-long term includes multiple diabetes programs surrounding next-generation insulin pumps and CGM/patch-pump technologies.
“We’re focused on the long term and intend to maintain important investments to deliver on this,” Martha said.

