Inovio’s smart vaccine delivery
Inovio Pharmaceuticals (NSDQ:INO) last summer picked up a contract from the U.S. government to develop a potential game-changer amid the COVID-19 pandemic.
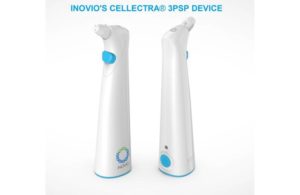
The U.S. Department of Defense awarded Inovio $71 million to support large-scale manufacturing for the Cellectra 3PSP smart device and the procurement of Cellectra 2000 devices, which are used to deliver Inovio’s INO-4800 COVID-19 vaccine candidate directly into the skin.
While the late-stage trial for the vaccine candidate is slated for later this year after the FDA put a temporary hold on it in the fall, the delivery method is still one to watch.
The small, portable, hand-held device runs on “AA” batteries and is designed to deliver the INO-4800 vaccine, meant to prompt the body’s immune system to drive an immune response.
Cellectra uses a brief electrical pulse to reversibly open small pores in the cell and allow plasmids to enter, which Inovio says is a limitation of other DNA and other nucleic acid approaches, such as mRNA. Once the DNA plasmids are inside the cell, they enable the cell to produce the targeted antigen, which is processed naturally to trigger the desired T-cell and antibody-mediated immune responses.
Inovio’s delivery system is designed to ensure that DNA medicine is efficiently delivered into the body’s cells to drive an immune response, while the medicines do not interfere with or change an individual’s own DNA in any way.

