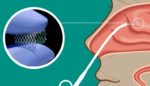
Lyra Therapeutics (Nasdaq:LYRA) announced today that it enrolled the first patient in its Phase 3 Enlighten II clinical trial. Enlighten II is evaluating the company’s LYR-210 in adult patients with chronic rhinosinusitis (CRS). Lyra’s proprietary XTreo platform delivers LYR-210. Watertown, Massachusetts-based Lyra designed the proprietary XTreo platform for precise, sustained and local delivery. The platform […]
Lyra Therapeutics
Lyra Therapeutics appoints new chief medical officer
Lyra Therapeutics (Nasdaq:LYRA) announced today that it appointed Dr. Richard Nieman as its new chief medical officer (CMO). Nieman’s appointment as CMO of Lyra, which develops the XTreo — a platform for enabling precise, sustained and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissues — will be […]
Lyra Therapeutics closes $100.5M private placement
Lyra Therapeutics (Nasdaq:LYRA) announced today that it closed its previously announced private placement worth $100.5 million. The company announced last week that, in the private placement, investors had the option in the private placement to purchase either shares of Lyra’s common stock at $4.22 per share or, in lieu thereof, pre-funded warrants to purchase shares […]
Lyra Therapeutics announces $100.5M private placement
Lyra Therapeutics (Nasdaq:LYRA) announced today that it agreed to sell securities in a private placement worth more than $100 million. The company — which develops the XTreo platform designed to enable precise, sustained, and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissues — did not list an […]
Lyra Therapuetics initiates Phase 3 trial for chronic rhinosinusitis treatment
Lyra Therapeutics (NSDQ:LYRA) announced today that it initiated the Phase 3 Enlighten I trial for its LYR-210 treatment. Watertown, Massachusetts-based Lyra’s Phase 3 Enlighten I clinical trial will evaluate LYR-210, delivered through Lyra’s proprietary XTreo drug-eluting implant, in adult, surgically naïve chronic rhinosinusitis (CRS) patients. The company designed LYR-210 for delivery in a brief, non-invasive, […]
Lyra Therapeutics appoints new CFO
Lyra Therapeutics (NSDQ:LYRA) announced today that it appointed Jason Cavalier as its new chief financial officer, effective today. Cavalier succeeds the company’s current CFO, Don Elsey, who is retiring while he remains expected to serve in an advisory role to assist with the transition. Watertown, Massachusetts-based Lyra — which develops the XTreo platform designed to […]
Lyra touts non-human trial of XTreo drug-eluting implant
Lyra Therapeutics (NSDQ:LYRA) is touting preclinical data for its XTreo drug-eluting implant for dosing in local sinus tissues. Watertown, Massachusetts-based Lyra’s preclinical results were published — “Drug Release and Pharmacokinetic Evaluation of Novel Implantable Mometasone Furoate Matrices in Rabbit Maxillary Sinuses” — were published online in the peer-review journal, American Journal of Rhinology & Allergy. The […]
Lyra inks $135M partnership to expand chronic rhinosinusitis treatment in Asia
Lyra Therapeutics (NSDQ:LYRA) announced today that it entered into a strategic partnership with LianBio for its LYR-210 treatment for chronic rhinosinusitis. Watertown, Mass.–based Lyra, which develops the LYR-210 therapeutic to be locally delivered by its proprietary XTreo platform, could receive up to $135 million in total payments through the strategic partnership and exclusive license agreement […]
Lyra Therapeutics rises on Q1 earnings
Lyra Therapeutics (NSDQ:LYRA) shares finished up on the day despite first-quarter results that missed the consensus earnings forecast. The Watertown, Mass.-based chronic rhinosinusitis (CRS) treatment developer posted losses of -$7.8 million, or -60¢ per share for the three months ended March 31, 2021, for a -84.4% bottom-line slide. Lyra’s EPS of -60¢ came in 48¢ […]
Lyra Therapeutics touts results from chronic rhinosinusitis treatment trial
Lyra Therapeutics (NSDQ:LYRA) presented positive results from a Phase 2 study of its therapeutic for treating chronic rhinosinusitis (CRS). Watertown, Mass.-based Lyra’s Phase 2 Lantern study evaluated its LYR-210 long-acting product for CRS, which is administered in-office to deliver a sustained release for up to six months as a non-invasive alternative to surgery for patients […]