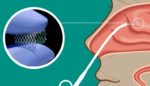
OM1 today announced positive results from its partnership with Medtronic (NYSE:MDT) evaluating the Propel corticosteroid-eluting implants. The Propel bioabsorbable implant family came to Medtronic through its acquisition of IntersectENT in 2022. OM1 and Medtronic partnered on their study to focus on healthcare resource use and long-term outcomes in patients with chronic rhinosinusitis (CRS) who underwent […]
Otolaryngology Ear, Nose & Throat
These ear tubes with liquid infused materials may improve ear infection treatment outcomes
A research collaboration in Boston may have uncovered a design overhaul to improve outcomes for ear tubes known as tympanostomy tubes (TTs). These TTs create an opening between the ear canal and middle ear. Their aim is to ventilate the middle ear, provide a route for fluid to drain out and allow antibiotic drops to […]
Lyra Therapeutics enrolls first patient in Phase 3 chronic rhinosinusitis trial
Lyra Therapeutics (Nasdaq:LYRA) announced today that it enrolled the first patient in its Phase 3 Enlighten II clinical trial. Enlighten II is evaluating the company’s LYR-210 in adult patients with chronic rhinosinusitis (CRS). Lyra’s proprietary XTreo platform delivers LYR-210. Watertown, Massachusetts-based Lyra designed the proprietary XTreo platform for precise, sustained and local delivery. The platform […]
Lyra Therapeutics appoints new chief medical officer
Lyra Therapeutics (Nasdaq:LYRA) announced today that it appointed Dr. Richard Nieman as its new chief medical officer (CMO). Nieman’s appointment as CMO of Lyra, which develops the XTreo — a platform for enabling precise, sustained and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissues — will be […]
Medtronic completes Intersect ENT acquisition
Medtronic (NYSE:MDT) announced that it completed its previously announced $1.1 billion acquisition of Intersect ENT (Nasdaq:XENT). Fridley, Minnesota-based Medtronic announced in August 2021 that it would acquire all outstanding shares of the Menlo Park, Calif.-based sinus implant maker for $28.25 per share in an all-cash transaction. As a result of the transaction, Medtronic acquires Intersect ENT’s Propel and Sinuva drug-eluting […]
Impel NeuroPharma rebrands to Impel Pharmaceuticals as part of corporate transformation
Impel NeuroPharma (Nasdaq:IMPL) announced today that it changed its name to Impel Pharmaceuticals as part of a corporate rebrand. Seattle-based Impel develops proprietary Precision Olfactory Delivery (POD) technology to treat patients suffering from diseases across a range of areas in addition to the central nervous system. The company’s Trudhesa nasal spray for treating migraine in […]
Lyra Therapeutics closes $100.5M private placement
Lyra Therapeutics (Nasdaq:LYRA) announced today that it closed its previously announced private placement worth $100.5 million. The company announced last week that, in the private placement, investors had the option in the private placement to purchase either shares of Lyra’s common stock at $4.22 per share or, in lieu thereof, pre-funded warrants to purchase shares […]
Lyra Therapeutics announces $100.5M private placement
Lyra Therapeutics (Nasdaq:LYRA) announced today that it agreed to sell securities in a private placement worth more than $100 million. The company — which develops the XTreo platform designed to enable precise, sustained, and local delivery of medications to the ear, nose and throat (ENT) passages and other diseased tissues — did not list an […]
Lyra Therapuetics initiates Phase 3 trial for chronic rhinosinusitis treatment
Lyra Therapeutics (NSDQ:LYRA) announced today that it initiated the Phase 3 Enlighten I trial for its LYR-210 treatment. Watertown, Massachusetts-based Lyra’s Phase 3 Enlighten I clinical trial will evaluate LYR-210, delivered through Lyra’s proprietary XTreo drug-eluting implant, in adult, surgically naïve chronic rhinosinusitis (CRS) patients. The company designed LYR-210 for delivery in a brief, non-invasive, […]
Lyra Therapeutics appoints new CFO
Lyra Therapeutics (NSDQ:LYRA) announced today that it appointed Jason Cavalier as its new chief financial officer, effective today. Cavalier succeeds the company’s current CFO, Don Elsey, who is retiring while he remains expected to serve in an advisory role to assist with the transition. Watertown, Massachusetts-based Lyra — which develops the XTreo platform designed to […]