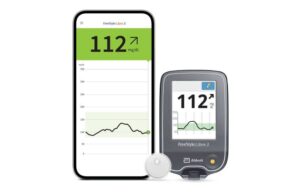
The FreeStyle Libre 3 reader pairs with the company’s latest continuous glucose monitor (CGM) sensor, cleared by the FDA last year. The addressable market for the sensor keeps growing, with massive coverage expansions all around the globe.
A Medicare coverage update kicked in this past spring, expanding access to the sensor. This modification includes people with diabetes who receive insulin treatment or have a history of problematic hypoglycemia.
According to Abbott, patients who may not have a compatible smartphone can use the reader. All other patients can continue to access glucose readings through the FreeStyle Libre 3 app. The sensor sends readings directly to the app on patients’ smartphones without requiring scans.
“FreeStyle Libre continuous glucose monitoring systems are prescribed by more doctors for their Medicare patients than any other system,” said Jared Watkin, SVP for Abbott’s diabetes care business. “With recent coverage expansion, there are another two million Medicare patients who manage their diabetes with insulin that are now eligible to use the FreeStyle Libre 3 system to track their glucose levels and have better health outcomes.”
A positive period for Abbott diabetes
The medtech giant’s diabetes business continues to drive growth and produce strong results. Reported as part of Abbott’s second-quarter earnings results, the unit brought in sales of $1.4 billion in the quarter. That was good for 19.4% growth year-over-year.
FreeStyle Libre continues to drive growth as CEO Robert Ford projected earlier this year. In addition to the U.S. Medicare expansion, last month, France authorized reimbursement to include all people who use basal insulin as part of their diabetes management.
Integration opportunities continue to arise for Libre, too. Ford expects the company to finalize Libre integration into Tandem Diabetes Care’s insulin pumps in the U.S. this year. The companies first struck a deal to combine technologies in 2020. Last month, Insulet announced progress in integrating FreeStyle Libre 2 into its Omnipod 5 insulin delivery system, too. Meanwhile, FreeStyle Libre 3 continuous glucose monitor (CGM) received U.K. authorization for use in automated insulin delivery this year.
The company also expects to begin a trial on a glucose-ketone dual-sensor trial in the fourth quarter. Abbott also plans to convert its FreeStyle Libre 2 users to streaming, switching from scans to real-time streaming through an app update. The first successful conversion to streaming recently took place in the U.K.
Ford says there’s “a lot of pipeline activity” slated for the second half of 2023.
