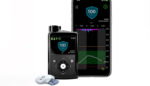
Abbott (NYSE:ABT) has made its FreeStyle Libre 3 reader available in the U.S., enabling people on Medicare to access the system. The FreeStyle Libre 3 reader pairs with the company’s latest continuous glucose monitor (CGM) sensor, cleared by the FDA last year. The addressable market for the sensor keeps growing, with massive coverage expansions all […]
Centers for Medicare and Medicaid Services (CMS)
Medicare now covers new Medtronic MiniMed 780G automated insulin pump
Medtronic (NYSE:MDT) announced today that Medicare now covers its new MiniMed 780G system with the Guardian 4 sensor. Coverage for the recently FDA-approved automated insulin delivery system extends to all eligible Medicare and Medicare Advantage beneficiaries. Medtronic intends to begin processing orders immediately. The company plans to start shipments to eligible recipients with type 1 […]
Dexcom, Abbott applaud Medicare expansion for CGMs
Dexcom (Nasdaq:DXCM) and Abbott (NYSE:ABT) both commended a change to Medicare policies that benefits continuous glucose monitor (CGM) users. Last month, the Centers for Medicare & Medicaid Services (CMS) published a final local coverage determination (LCD) expanding coverage for CGMs. The ruling has now gone into effect. CMS first published the LCD modifying coverage criteria for […]
Expanded Medicare coverage for CGM to go into effect next month
The Centers for Medicare & Medicaid Services today published a final local coverage determination (LCD) expanding coverage for CGMs. New, expanded coverage of continuous glucose monitors (CGMs) under Medicare go into effect in April. CMS first published the LCD modifying coverage criteria for CGMs in October. The modification includes people with diabetes who receive insulin […]
Medicare to cover Dexcom G7 when it launches this week
Dexcom (Nasdaq:DXCM) announced today that Medicare offers coverage for its beneficiaries using the next-generation G7 CGM. The company announced the coming U.S. launch for G7 in a Super Bowl Commercial with musician Nick Jonas last night. It’s slated to roll out on Friday, Feb. 17. San Diego-based Dexcom met the category requirements for therapeutic continuous […]
Analysts: Abbott, Dexcom to benefit from proposed CMS decision on CGMs
Analysts see a new proposed local coverage determination (LCD) from CMS as a potential boost for Dexcom (Nasdaq:DXCM) and Abbott (NYSE:ABT). The Centers for Medicare and Medicaid Services (CMS) yesterday published the LCD modifying coverage criteria for continuous glucose monitors (CGMs). The modification includes people with diabetes who receive insulin treatment or have a history […]
Analysts say high payor coverage, increased CGM adoption bodes well for Abbott, Dexcom
Analysts have suggested that companies like Abbott (NYSE:ABT) and Dexcom (Nasdaq:DXCM) may benefit from expected reimbursement progress. BTIG hosted a conference call with North Shore Medical Center (Salem, Massachusetts) Medical Director Dr. Gary Cohen and Healthcare Analytics, LLC analyst and consultant Dr. Joshua Cohen. The two experts offered insights into prescriber and patient interest in […]
Medtronic announces recent reimbursement wins for CGMs in a number of geographies
Medtronic (NYSE:MDT) announced today that reimbursement for its CGMs has been expanded or initiated across a number of countries. Earlier this month, the Ontario, Canada provincial government announced a comprehensive reimbursement program for continuous glucose monitoring (CGM) for eligible residents who have type 1 diabetes, adding to a similar program for those with type 1 […]
CMS expands Medicare coverage to CGMs that integrate with Medtronic insulin pumps
Medtronic (NYSE:MDT) announced today that the U.S. Centers for Medicare & Medicaid Services (CMS) will expand coverage for CGMs. CMS’ expanded coverage covers all types of CGMs (continuous glucose monitors), including adjunctive and non-adjunctive CGMs, and it notably covers CGMs that integrate with Medtronic insulin pumps. According to a news release, the proposed rule was […]
Massachusetts offers Medicaid coverage for Pear Therapeutics’ digital therapeutics
Pear Therapeutics announced today that MassHealth intends to cover the company’s prescription digital therapeutics (PDTs). MassHealth, Massachusetts’ Medicaid program, notified Pear of its plans to cover the FDA-authorized reSET and reSET-O PDTs for treating substance use disorder and opioid use disorder, respectively. The decision remains subject to certain customary conditions, including federal and state approvals as […]