NEWS RELEASE: ProMed Pharma celebrates one-year anniversary of new Maple Grove development and analytical laboratories ProMed Pharma recently celebrated the first anniversary of the opening of its Product Development and Analytical Testing facility in Maple Grove, Minnesota. The facility currently includes two state-of-the-art Class 7 clean rooms with dedicated air handling, gowning areas and a […]
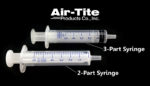
What is the difference between two-part and three-part syringes?
Syringes remain an integral part of the medical industry, yet they are often an afterthought in the R&D or production phase. William Foley, Air-Tite Products With the wide variety of options available on the market containing fundamental differences in design and composition, can the syringe selection affect the end-product? It’s possible to manufacture a syringe […]
Nemera’s Innovation Center reaches 100 employees
(Press Release) – Nemera‘s celebrated an important milestone: its Innovation center has just hired its 100th employee. The Innovation Center, a multi-discipline drug delivery device development expertise center, was created in 2008. Early on, it included a few engineers, who worked on product development. Over the last 10 years, it has grown consistently to reach […]
VIVA 2018 Roundup: Surmodics reports positive one-year data for SurVeil drug-coated balloon
By Sarah Faulkner & Fink Densford Surmodics (NSDQ:SRDX) touted data this week from an early feasibility study of the company’s SurVeil drug-coated balloon in patients with symptomatic peripheral artery disease due to de novo lesions of the femoral and popliteal arteries. All of the study’s 13 subjects met the acute success measures of safety at […]
Materials for needle-free injection technology: What you need to know
Specially formulated polycarbonates help advance drug delivery innovation that improves patient experience and promotes better medication compliance. Douglas Hamilton, Covestro Diabetes is on the rise. The International Diabetes Federation (IDF) estimates there are roughly 415 million people affected by this chronic condition. By 2045, this is expected to rise to 629 million. For many, living with diabetes requires […]
Lubrizol LifeSciences & Particle Sciences to host micro- & nano-particulate drug product development seminar
[PRESS RELEASE] – The Lubrizol Corporation announces its LifeSciences Pharmaceutical business will organize the first annual “Complex Drug Delivery Seminar: Micro- and Nano- Particulate Drug Product Development and Manufacturing” in Amsterdam on Thursday, September 27, 2018. Lubrizol LifeSciences through its pharmaceutical CDMO, Particle Sciences, and Co-Sponsor, Corbion, a world leader in bioresorbable polymers, would like to invite […]
Clinical Study Requirements: Drugs vs. Devices
Whether developing a new drug or medical device, designing and running clinical trials can be daunting. How drugs and devices are developed differ dramatically. Study design and regulatory pathways differ; requirements for study sites, end users, training, endpoints and follow up are all variables. This paper examines the key differences and how those impact clinical […]
From Zero to Six Million – Using a Component Management Process to Scale Up Manufacturing of Drug Delivery Devices
Drug delivery devices constitute one of the most rapidly growing segments of the medical device manufacturing market. Makers focus substantial R&D efforts on designing delivery mechanisms for new and existing drug formulations. However, even good initial designs can’t guarantee that all the parts for these devices can be manufactured correctly and assembled consistently, with few […]
PsiOxus Therapeutics shuffles the deck
PsiOxus Therapeutics shuffles the deck PsiOxus said it named Dr. Charles Morris as its chief development officer and Karen LaRochelle as its chief business officer. Morris comes from ImmunoGen, where he was chief development officer, bringing its lead candidate to Phase III development. Most recently, LaRochelle was the executive director of global business development at […]
Mylan CEO to testify before House panel over EpiPen pricing
(Reuters) –Mylan (NSDQ:MYL) Chief Executive Officer Heather Bresch will appear at a Sept. 21 congressional hearing over price increases for its EpiPen emergency allergy treatment, the U.S. House of Representatives Oversight Committee said in a statement on Wednesday. Mylan has been widely criticized, including by U.S. Democratic presidential candidate Hillary Clinton, for sharply raising the price […]