Vaxess Technologies announced today that it raised an additional $9 million in venture capital funding to support its vaccine delivery technology. Cambridge, Massachusetts-based Vaxess develops a shelf-stable vaccine patch with the potential for self-application. The company’s MiMix system features sustained-release technology for administering vaccines and therapeutics. For vaccines, its controlled release simulates the pace of […]
Pharmaceuticals
Analysts: GLP-1 drugs should have minor impact on insulin pump market — but will boost CGMs
Based on conversations with diabetes experts, analysts are playing down the potentially negative impact of GLP-1 receptor agonists on the diabetes technology industry. The GLP-1 drug class, which includes Ozempic and Wegovy, has cast a shadow of doubt over diabetes technology of late. This therapeutic class, a glucagon-like peptide 1, has proven to lead to […]
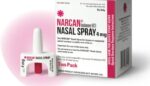
Emergent BioSolutions launches over-the-counter Narcan nasal spray
Emergent BioSolutions (NSYE:EBS) announced today that it plans to put its over-the-counter Narcan nasal spray on U.S. shelves next month. FDA approved the over-the-counter Narcan spray in May. Approval enabled direct-to-consumer sales in places like drug stores, convenience stores and more. The FDA nod came about six weeks after an agency committee voted unanimously in favor […]
Vaxxas wins $3.7M award to support needle-free typhoid vaccine tech
Vaxxas announced that it received a $3.67 million award to conduct studies of a typhoid vaccine delivered by its needle-free device. The company develops a vaccine patch based on its proprietary HD-MAP delivery technology. Vaxxas designed its HD-MAP technology to use an ultra-high-density array of micro projections, invisible to the human eye. Applied to the […]
BioCorRx submits implantable opioid use disorder treatment to FDA for expanded access
BioCorRx announced today that it submitted to the FDA an expanded access program application for its BICX104 implantable naltrexone pellet. BICX104, a biodegradable implantable pellet, treats opioid use disorder (OUD). The expanded access program would enable the treatment to reach eligible patients before full FDA approval. The FDA program provides access to products before approval […]
Emergent BioSolutions to cut 400 jobs, focus on Narcan nasal spray
Nasal Narcan spray maker Emergent BioSolutions (NSYE:EBS) announced a change in strategy and investment that affects 400 jobs. The Gaithersburg, Maryland-based company decided to reduce investment in and de-emphasize focus on growth in its CDMO business. This results in a reduction of operations at the company’s Bayview facility in Baltimore. Additionally, in response to changes […]
Kindeva wins £33M grant from UK to enhance drug-delivering inhalers
Kindeva Drug Delivery announced that it received a grant from a UK fund worth a $42.2 million (£33 million) investment. The grant from the UK Government’s Life Sciences Innovation Manufacturing Fund (LSIMF) aims to help Kindeva enhance inhaler manufacturing and sustainability. Funds improve Kindeva’s capability and capacity to manufacture the next generation of green pressurized […]
FDA approves another over-the-counter Naloxone nasal spray
Harm Reduction Therapeutics announced that it received FDA approval for its over-the-counter RiVive naloxone nasal spray. Pittsburgh-based HRT designed the 3 mg naloxone HCl nasal spray for the emergency treatment of opioid overdose. Approval helps make free or low-cost OTC nasal spray widely available in the U.S. The FDA first approved Narcan 4 mg OTC […]
UroGen to raise $120M to support sustained release hydrogel delivery platform
UroGen Pharma (Nasdaq:URGN) announced that it entered into a securities purchase agreement worth approximately $120 million. The placement includes the issuance of nearly 12.6 million ordinary shares or pre-funded warrants priced at $9.54 per share. UroGen expects the placement to close on or about today, subject to customary closing conditions. RA Capital Management and Great […]
RenovoRx, Imugene collab on oncolytic virus therapy delivery
RenovoRx (Nasdaq:RNXT) announced that it entered into a strategic research collaboration with Imugene to optimize oncolytic virus therapy delivery. Utilizing RenovoRx’s TAMP (Trans-Arterial Micro-Perfusion) therapy platform), the companies hope to enhance the treatment of difficult-to-access tumors. “Our collaboration with Imugene is an important milestone for RenovoRx as we expand our pipeline from exclusively treating locally […]