(Image from Lyra Therapeutics)
With the use of anti-inflammatory drugs constituting the standard of care for treating rhinosinusitis, Lyra Therapeutics sees an opportunity.
Whatever treatments are out there for the disease in which cavities around the nasal passages are inflamed, they leave room for improvement.
“We focused on chronic rhinosinusitis because it’s an area that has been ignored,” Lyra CEO Maria Palasis told Drug Delivery Business News. “It’s been called an unrecognized epidemic. It’s a disease where there, there just aren’t a lot of treatments.”
Watertown, Mass.-based Lyra has taken the initiative with its XTreo platform and the LYR-210 therapeutic.
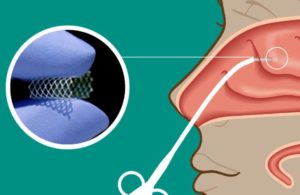
XTreo is what Palasis called an “industrial platform,” comprised of a flexible mesh capable of taking a high payload of a drug. The platform provides a consistent release of the therapeutic over time, with Lyra touting capabilities of six months of drug delivery.
Nasal sprays are used for the disease which recently appointed Lyra chief medical officer Dr. Robert Kern said affects 30 million people in the country, although it can be lived with.
Kern pointed out that patients dealt with antibiotics or surgery before the nasal steroids came along, but a problem came about with spray going down patients’ throats. The elimination of that issue, along with the longevity provided by XTreo, has Lyra excited about the improvements the platform offers.
“This device is an opportunity to take that nasal spray and shove it in somebody’s nose,” Kern said. “It gives off that steroid for six months. That’s a tremendous leap forward.”
Traditional steroid treatments offer between one and two weeks of relief from rhinosinusitis, Kern said, while surgery has between a 20% and 30% revision rate at five years.
Palasis noted that an estimated that, of about 8 million U.S. patients treated in a year, about 50% receive failed medical management, leading to surgery as the only option, with about 400,000 surgeries taking place in the U.S. each year.
“Surgery is not curative,” Palasis said. “It doesn’t address the underlying disease. We’re really trying to provide another option to physicians and patients prior to surgery.”
While Kern said it’s difficult to compare “apples to oranges” with regard to Lyra’s treatment versus the standard options, it’s clear that there is a groundbreaking element to XTreo and the LYR-210 therapeutic.
“This drug makes a huge difference — a difference at the caliber of the level of surgery,” Kern said. “We’re not in the endzone yet, but this has the potential to really be a game-changer.”
LYR-210 is designed for patients who have not had a prior surgery, while the company is also developing LYR-220 for those who have had surgery. Lyra intends to introduce LYR-220 into the clinic this year, Palasis said.
The company has collected data from the Phase 2 trial for LYR-210 and will meet with the FDA ahead of a Phase 3 study, while a Phase 2 study for LYR-220 is slated for this year as well.
“We are moving as quickly as we can,” Palasis said. “Right now, we’re focused on getting LYR-210 into the Phase 3 trial and LYR-220 into the Phase 2, but we’re certainly very motivated to move quickly and get these therapies to patients.”
With plenty on the company’s plate for the immediate future, there is still some consideration for more applications of its technologies down the line.
The company is conducting market research in a few different areas, Palasis said, with the ear being one area in particular. Lyra is looking at making its technology even smaller to fit into the structures of the ear to provide treatments through that avenue.
Additionally, Palasis noted that the company is exploring the possibility of delivering drugs from the nose to the brain, with delivery to the olfactory cleft a possibility.
For now, though, the focus remains on delivering the alternative to steroids and surgery that offers a combination of simplicity and effectiveness in treating rhinosinusitis.
“The beauty of this device is that it can be put in at any office easily,” Kern said. “It’s very well tolerated and it’s an office visit, not an operation. … There are many, many benefits that could come from this.
“I don’t really think the number of surgeries will go down. I think the number of patients we treat and make better will be enhanced.”

