The all-in-one, disposable CGM features a simple, two-step insertion process for people with diabetes using multiple daily injections (MDI). Medtronic’s newest no-fingerstick sensor eliminates the need for overtape, too. It seamlessly integrates with the InPen smart insulin pen for real-time, personalized dosing guidance to simplify diabetes management.
Medtronic plans to begin a phased launch for Simplera at the European Association for the Study of Diabetes (EASD) 59th Annual Meeting. It takes place in Hamburg, Germany, from Oct. 2-6.
Commercializing a new CGM comes at a good time for Medtronic, which could see the benefits from the newly popular GLP-1 drug class. Analysts recently outlined the ways in which those drugs may provide a boom for the CGM market.
It also gives the company a new offering to rival the combination of Abbott and Bigfoot Biomedical. The two companies already combine the FreeStyle Libre CGM and Bigfoot Unity smart insulin pen cap system and Abbott just acquired Bigfoot earlier this month.
“Despite the rapid adoption of CGM over the past decade, less than 30% of individuals on MDI therapy using a CGM achieve glycemic targets — highlighting a significant unmet need. We’re excited to help more people to reach their goals with our advanced algorithm in InPen powered by our smallest and most comfortable CGM to date,” said Que Dallara, EVP and president, Medtronic Diabetes. “This newest addition of a Smart MDI solution to our holistic portfolio demonstrates our commitment to meeting people where they are in their diabetes journey with simplified solutions that help make life with diabetes easier.”
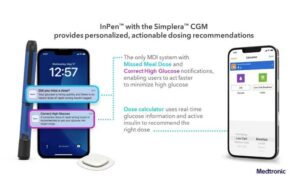
More about the Simplera CGM and Smart MDI system
The Smart MDI system referenced by Dallara integrates real-time CGM with the InPen smart insulin pen. An adjustable algorithm powers the system to deliver personalized dosing recommendations.
InPen combined with Simplera provides users with actionable insights to reduce guesswork and manual calculations. Medtronic says this helps to simplify diabetes management.
(Read also: InPen was included in a class-action lawsuit alleging third-party patient data sharing)
Simplera holds an indication for people ages two and up and has compatibility with iOS and Android operating systems. It remains investigational in the U.S. Medtronic currently has its automated insulin delivery system integrated with Simplera under review for CE mark.
Medtronic CEO Geoff Martha has previously mentioned the next-generation Simplera CGM. Half the size of the current Guardian 4, the disposable Simplera could theoretically be Medtronic’s counter to the existing CGMs on the market made by the likes of Abbott and Dexcom.
When asked about this CGM at the Goldman Sachs global healthcare conference in June, Martha confirmed that the company made its regulatory submissions recently. Medtronic now has CE mark after applying for it “months ago.” It also submitted for FDA approval within a month of that June conference. Martha offered no timeline for the approvals but set his sights on a fall launch in Europe, which has now been confirmed.
“It is half the size of our current sensor, and it takes two seconds to put on,” Martha said at Goldman Sachs. “I actually went through our clinical trial, nondiabetic. But I wanted to see, and it’s very easy to use. We think that’s coming soon. And it’ll have an even bigger impact.”
A strong stretch for Medtronic Diabetes continues
To cap off fiscal 2023 in April, Medtronic received FDA approval for its next-generation MiniMed 780G automated insulin delivery system. Not long after, the company fully resolved a warning letter issue with the FDA.
Medtronic began the first quarter of 2024 with its Diabetes unit helping to drive the company’s overall growth. Highlights included the U.S. launches for the MiniMed 780G automated insulin delivery system, which led to led to 6.8% revenue growth year-over-year for Medtronic Diabetes.
The quarter also brought news of a planned $738 million acquisition of insulin delivery technology developer EOFlow.
On the company’s earnings call last month, Martha highlighted the continued success for the once-maligned Diabetes unit.
“The turnaround in Diabetes is real and underway, and I am pleased with the progress the team is making,” he said. “And we’re just at the beginning of this inflection point for the business.”

