 Researchers from the University of Massachusetts Amherst have designed a nanoparticle delivery system to assist the gene-editing tool known as CRISPR/Cas9 across the cell membrane and into the cell nucleus, effectively avoiding the cell’s natural defense mechanisms. The team’s work was published in ACS Nano.
Researchers from the University of Massachusetts Amherst have designed a nanoparticle delivery system to assist the gene-editing tool known as CRISPR/Cas9 across the cell membrane and into the cell nucleus, effectively avoiding the cell’s natural defense mechanisms. The team’s work was published in ACS Nano.
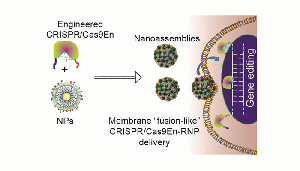
“CRISPR has two components: a scissor-like protein called Cas9, and an RNA molecule called sgRNA that guides Cas9 to its target gene. Once the Cas9-sgRNA pair gets to the destination gene in the nucleus, it can interrogate its genetic mistakes and correct them with the help of the host cell’s repair machinery,” principal investigator Rubul Mout said in prepared remarks.
Since CRISPR was discovered in 2012, gene editing has emerged as a means to treat otherwise incurable genetic diseases by manipulating diseased genes. “However, to achieve this, biotech and pharmaceutical companies are constantly searching for more efficient CRISPR delivery methods,” Mout added.
The team of researchers engineered the Cas9 protein, Cas9En, and carrier nanoparticles to develop a delivery system. “By finely tuning the interactions between engineered Cas9En protein and nanoparticles, we were able to construct these delivery vectors,” co-principal investigator Vincent Rotello explained. “The vectors carrying the Cas9 protein and sgRNA come into contact with the cell membrane, fuse, and release the Cas9:sgRNA directly into the cell cytoplasm.”
“Cas9 protein also has a nuclear guiding sequence that ushers the complex into the destination nucleus. The key is to tweak the Cas9 protein,” he added. “We have delivered this Cas9 protein and sgRNA pair into the cell nucleus without getting it trapped on its way. We have watched the delivery process live in real time using sophisticated microscopy.”
With their nanoparticle delivery system, the team claimed they could deliver the Cas9 protein and sgRNA pair into 90% of cells with an editing efficiency of 30%.
“90% cytosolic/nuclear delivery is a huge improvement compared to others methods,” Mout said.
The team’s engineered nanoparticle system could serve to deliver other materials including polymers, lipid nanoparticles or self-assembling peptide, the team suggested.
“Now that we have achieved efficient gene editing in cultured cells, we are aiming to edit genes in pre-clinical animal models. We are also interested in gene editing for adoptive therapies, where a diseased cell is isolated from a patient, corrected by CRISPR in the lab, and delivered back to the patient,” Rotello said.

