Toleikis spent 13 years leading the Cell Pouch System developer as president and CEO. He remains an executive at the company as the planned transition moves him to chief technology officer (CTO). Additionally, Toleikis will remain CEO during the company’s search period.
His responsibilities include continuing to work closely with executive chair Frank Holler. Holler aims to take a leadership role and work closely with Toleikis. Together they plan to advance Sernova’s development during the search, according to a news release. Sernova engaged Slone Partners, an executive search firm, to assist its board in the hiring of the new CEO.
Toleikis recently spoke to Drug Delivery Business News about how the company seeks to treat insulin-dependent diabetes. Read here.
“On behalf of the board, I am very grateful for the substantial contributions made by Dr. Toleikis to the company’s growth and success over the years,” said Holler. “Philip has played a pivotal role in strategically advancing Sernova’s CPS platform to become one of the world’s most prominent cell therapy platforms and a leader in the development of cell therapy treatments for insulin-dependent diabetes. With the company’s anticipated next phase of growth in U.S. capital markets, it is a natural time to recruit a new CEO.”
About Toleikis and Sernova
Toleikis joined Sernova in 2009. Under his leadership, the company developed its Cell Pouch System to its current state in ongoing Phase 1/2 type 1 diabetes clinical trials. Holler said the move to CTO enables him to focus on accelerating the system’s development.
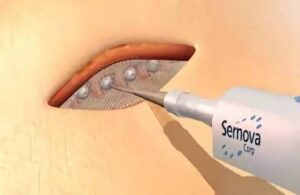
London, Ontario-based Sernova designed its proprietary Cell Pouch System as an implantable and scalable medical device. It forms a natural environment in the body for the long-term survival and function of therapeutic cells. Those cells release necessary proteins or factors missing from the body to treat chronic diseases, including insulin-dependent diabetes.
Sernova’s ongoing Phase 1/2 clinical trial evaluates six patients with long-standing, insulin-dependent T1D. Those patients have hypoglycemia unawareness prior to treatment. Sernova announced positive data from that trial in June. That includes three patients achieving insulin independence.
Earlier this month, the company received approval to proceed with a strategically optimized protocol reducing the time required for patient treatment. It also accelerates potential secondary endpoint efficacy achievement with more optimal dosing.
I am very proud of the company’s achievements to date, and I look forward to working closely with the new CEO,” said Toleikis. “I am delighted to continue working with our extraordinary team, and the best-in-class leadership within, to advance Sernova to the next level of growth as well as the continued development of Sernova’s cell therapy platform on the world stage.”

