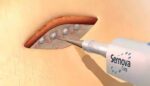
Sernova announced today that it entered into a preclinical research collaboration on drug delivery with AstraZeneca. The collaboration aims to evaluate the use of Sernova’s Cell Pouch System in combination with AstraZeneca’s novel therapeutic cells. London, Ontario-based Sernova designed its proprietary Cell Pouch System as an implantable and scalable medical device. It forms a natural […]
AstraZeneca plc
Viatris, Kindeva win FDA approval for first generic version of Symbicort inhalation aerosol
Viatris (Nasdaq:VTRS) and Kindeva Drug Delivery announced today that the FDA approved its generic version of AstraZeneca’s Symbicort. In March 2021, the FDA granted tentative approval to Breyna (Budesonide and Formoterol Fumarate Dihydrate Inhalation Aerosol) — the first generic version of Symbicort. Today, the agency granted approval for the abbreviated new drug application (ANDA) for […]
Pharma 50: Here’s how the world’s largest pharma companies are doing
The global pharmaceutical industry held up well during the pandemic, with 10 of the largest businesses only seeing a roughly –3% drop in revenue in 2020. Eight of the 10 even came out ahead. That’s one of the big takeaways from our sister publication Drug Discovery & Development’s inaugural Pharma 50, a compilation of data […]
FDA tentatively approves first generic version of Symbicort inhalation aerosol
Viatris (NSDQ:VTRS) and Kindeva Drug Delivery announced today that the FDA granted tentative approval of the first generic version of Symbicort. Symbicort, an inhalation aerosol is indicated for certain patients with asthma or chronic obstructive pulmonary disease (COPD). The drug had U.S. branded sales of $3.5 billion for the 12 months ended January 2021, according […]
AbbVie gains U.S. nod for $63B Allergan takeover
Pharmaceutical giant AbbVie (NYSE:ABBV) has won U.S. antitrust approval for its purchase of Allergan (NYSE:AGN) for $63 billion in cash and stock. The purchase is seen as a hedge against the impending expiration of U.S. patents on AbbVie’s blockbuster drug, Humira. North Chicago-based AbbVie announced the deal in June 2019, but ran up against U.S. Federal Trade Commission […]
Rani debuts human trial data for robotic pill tech
Rani Therapeutics this week touted the results of a first-in-human feasibility trial for its swallowable robotic pill, the RaniPill. Rani has developed a capsule that delivers an intestinal injection of medicine without exposing the drugs to digestive enzymes. The RaniPill device travels through the stomach and eventually reaches the intestine, where it injects the drug […]
Pharma stocks fall as Rep. Cummings launches drug pricing investigation
Chairman of the House Oversight Committee Rep. Elijah Cummings (D-MD) launched an investigation today into the drug pricing practices of 12 pharmaceutical companies. A number of companies named in the investigation saw their stock prices fall, including Amgen (NSDQ:AMGN), AbbVie (NYSE:ABBV), AstraZeneca (NYSE:AZN), Eli Lilly (NYSE:LLY) and Novo Nordisk (NYSE:NVO). A hearing is reportedly set for Jan. 29 and will […]
AstraZeneca touts long-term safety, efficacy data for asthma biologic
AstraZeneca (NYSE:AZN) today touted results from a Phase III extension trial evaluating the long-term safety and efficacy of benralizumab as an add-on maintenance therapy for patients with severe eosinophilic asthma. Benralizumab, or Fasenra, is approved as an add-on maintenance treatment for severe eosinophilic asthma in an array of countries, including the U.S., the E.U., and Japan. […]
FDA warns of flesh-eating genital infection linked to diabetes drug
The FDA this week warned of serious flesh-eating bacterial infections, or necrotizing fasciitis, reported by patients taking sodium-glucose cotransporter-2 (SGLT2) inhibitors intended for treating type 2 diabetes. The federal watchdog said that between March 2013 and May 2018, 12 cases of Fournier’s gangrene, a rare but life-threatening bacterial infection of the tissue under the skin […]
Adherium looks to give doctors a window into their patients’ lives
To control an asthma patient’s symptoms, doctors will prescribe an inhaler to stop symptoms from cropping up and a rescue inhaler to be used if patients find themselves in the middle of an asthma attack. “But if your rescue inhaler doesn’t give you the relief that you need, your next recourse is to go to […]