BD [WtwhTicker symbol=”BDX”](NYSE: BDX)[/WtwhTicker] issued an urgent field safety notice in the UK to warn of potential issues with its Alaris neXus V700 infusion pump system. According to the notice, BD identified that the anti-free flow clamp may not activate when the system door opens. This allows for the potential uncontrolled flow of medication. The […]
BD
BD ups domestic syringe production in wake of FDA warnings on syringes made in China
BD (NYSE:BDX) announced today that it is increasing its U.S. syringe production after the FDA issued warnings related to syringes manufactured in China. In November, the FDA warned of the potential for device failures with plastic syringes manufactured in China. Failures could include leaks, breakage and other problems. The agency said it received information about quality […]
BD inks $85M settlement over Alaris defect disclosure claims
BD (NYSE:BDX) announced that it filed settlement documents for an $85 million agreement to resolve claims regarding its Alaris infusion pump technology. The Alaris system has been much maligned over the past few years. Its troubles date back to a Class I recall in early 2020. The recall, which centered around multiple system errors, software […]
BD recalls more Alaris pumps due to compatibility issues with Cardinal Health syringes
The FDA determined that another recall of BD (NYSE:BDX) Alaris infusion pumps is Class I, the most serious kind. In the latest Alaris recall, the company cites compatibility issues with Cardinal Health Monoject syringes. The issue mainly relates to changes made to the products by Cardinal Health. It affects more than 1 million total devices. […]
Embecta increases 2023 guidance, reports closed-loop insulin delivery tech progress
Embecta (Nasdaq:EMBC) shares rose this morning as it increased its full-year guidance and shared an update on its automated insulin delivery technology. The company also reported third-quarter results that came in ahead of the consensus forecast. Shares of EMBC ticked up 3.1% at $22.27 apiece in early-morning trading today. MassDevice’s MedTech 100 Index — which […]
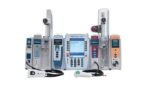
FDA clears updated BD Alaris infusion system after long-term commercial hold
BD (NYSE:BDX) announced today that the FDA granted 510(k) clearance for its updated Alaris infusion system. Shares of BDX closed out Friday evening priced at $264.84 each. They ticked up significantly after market close on the back of the Alaris news, rising 5.9% to $280.40 apiece. Clearance enables both remediation and a return to full […]
8 drug delivery innovations you should know
Innovations in drug delivery never stop, and over the past 12 months or so a wide variety have been in the spotlight. Constant progress continues in the diabetes space, both in the form of insulin delivery and other drug delivery methods. Elsewhere, we see implants, patches, syringes and more showcasing the many ways we can […]
BD discloses 8 cybersecurity vulnerabilities with Alaris infusion system
BD (NYSE:BDX) today voluntarily posted a product security bulletin for a number of vulnerabilities with its Alaris infusion system. Franklin Lakes, New Jersey-based BD recently identified eight vulnerabilities. These vulnerabilities are associated with the BD Alaris system with Guardrails Suite MX, versions 12.1.3 and earlier. The company discovered the vulnerabilities through routine internal security testing […]
BD is cutting 60 jobs at Ireland syringe manufacturing facility
BD (NYSE: BDX) announced plans to eliminate 60 positions at its manufacturing facility based in Drogheda, Ireland. A statement provided by a BD spokesperson confirmed a targeted reduction of 60 positions due to a combination of factors. These include the effects of the COVID-19 pandemic and the company’s spinoff of its diabetes business, Embecta. The […]
BD continues to expand in Ireland
BD (NYSE:BDX) announced that it opened a new facility in Ireland and committed a further investment within the country. Last week, the company held a grand opening ceremony for a new $4.3 million (€4 million) R&D facility in Blackrock, Dublin. BD also announced an additional $32.1 million (€30 million) investment to expand its Enniscorthy, Wexford […]