 Innovations in drug delivery never stop, and over the past 12 months or so a wide variety have been in the spotlight.
Innovations in drug delivery never stop, and over the past 12 months or so a wide variety have been in the spotlight.
Constant progress continues in the diabetes space, both in the form of insulin delivery and other drug delivery methods. Elsewhere, we see implants, patches, syringes and more showcasing the many ways we can deliver therapeutics.
About a year ago, we clued in readers to a list of six drug delivery innovations they should know. Here, we provide a couple of updates on those and try to introduce a few more developments that you should know:
The latest in automated insulin delivery tech
Automated insulin delivery represents one of the most high-profile markets in drug delivery, with the technology providing potentially life-saving therapy for people with diabetes.
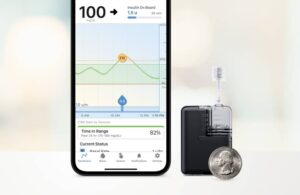
There’s still plenty of innovation going on in this market, and a few more automated insulin delivery systems made waves since.
Earlier this month, Tandem Diabetes Care won FDA clearance for its new Tandem Mobi automated insulin delivery system. Tandem says Mobi, which is fully controllable from a mobile app, is the world’s smallest durable AID system. It features a 200-unit insulin cartridge and an on-pump button to provide an alternative to phone control for insulin boluses. It comes in at less than half the size of the existing Tandem pump system, the t:slim X2 pump.
In May, the FDA cleared the Beta Bionics iLet ACE automated insulin pump and iLet dosing decision software. Beta Bionics’ iLet Bionic Pancreas uses an adaptive, closed-loop algorithm. It initializes with the user’s body weight and requires no additional insulin dosing parameters. The algorithm removes the need to manually adjust insulin pump therapy settings and variables.
While Omnipod 5 marked Insulet’s progress in automated insulin delivery last year, the company is already picking up steam with another offering. In April, the FDA cleared Omnipod GO — a long-acting insulin delivery system for the basal-only insulin population.
Keep an eye out for Tidepool Loop (partnered with BD diabetes spinoff Embecta) and Ypsomed (partnered with Abbott and Dexcom CGMs).
Insulin pen add-ons

Insulin injector pens are one big piece of the market, and two recent innovations improving them are worth touching on.
In December 20222, Biocorp won FDA 510(k) clearance for its Mallya device that connects insulin pens. The Mallya smart sensor attaches directly to insulin pen injectors to make them connected devices. It automatically collects and records key treatment information and transmits it to a dedicated digital application. That data includes selected insulin units, date and time of injection.
Innovation Zed makes a similar device, receiving CE mark approval in February for InsulCheck Dose. The company designed InsulCheck Dose as a single-unit add-on device for pen injectors. It automatically captures dose value dialed, injection event time stamps and temperature. The device also captures mounting and unmounting events.
Alternative diabetes therapies utilizing drug delivery methods
Aside from insulin delivery, companies are developing innovative alternatives to manage and treat diabetes.
Vivani Medical’s NanoPortal technology is an implant for steady medication delivery over extended periods of time. The company aims to guarantee correct doses for patients while mitigating safety concerns around fluctuating drug release profiles. The technology can also deliver large hydrophilic molecules, including peptides and proteins. The company believes this enables a broader range of therapeutic applications.

Vivani’s targeted conditions for this technology include type 2 diabetes, non-alcoholic steatohepatitis (NASH) and obesity.
Sernova has been making progress on its Cell Pouch System, an implantable and scalable medical device that forms a natural environment in the body for the long-term survival and function of therapeutic cells. Those cells release necessary proteins or factors missing from the body to treat chronic diseases, including insulin-dependent diabetes.
Keep your eyes out for a collaboration between Humacyte and JDRF, which are developing a biovascular pancreas (BVP).
They aim to advance the development of Humacyte’s BVP for treating type 1 diabetes. Humacyte designed its BVP to deliver insulin-producing islets using its investigational, tissue-engineered blood vessel. The company calls it the Human Acellular Vessel (HAV).
Ear tubes with liquid-infused materials for treating ear infections
A research collaboration found a potential drug delivery innovation to improve outcomes for ear tubes known as tympanostomy tubes (TTs). This research came out of the Wyss Institute for Biologically Inspired Engineering at Harvard University, Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), and Massachusetts Eye and Ear (MEE) in Boston.
Using TTs fabricated from a liquid-infused material — called iTTs — researchers co-optimized difficult-to-reconcile functions. These include fast drug delivery into the middle ear and fast drainage out, resistance against water crossing from the outside into the middle ear, and preventing bacterial and cell adhesion to tubes.
The researchers used chinchillas for an in vivo model relevant to the human ear to compare the iTTs’ performance against traditional TTs. Chinchillas are the gold standard for studying middle ear diseases and treatments, the team said, because chinchillas and humans have a similar frequency range of hearing and similarly sized tympanic membranes.
The ITTs preserved hearing and enabled easy and reliable dosing of antibiotic ear drops to the middle ear compared to conventional tubes, the researchers said.
Micron Biomedical’s needle-free drug delivery tech

The patented microarray technology enables the administration of drugs and vaccines that require cold storage and administration within minutes. It can be self-administered or caregiver-administered.
Micron says its technology simplifies transport, storage and administration while eliminating traditional injection sharps waste. Completed trials demonstrated clinical efficacy and strong patient preference, according to the company.
In May, Micron announced that it added $3 million to its Series A fundraising round, bringing the round’s total to $17 million.
MIT’s transdermal drug delivery patch
Researchers at MIT potentially found a way to address the difficulties of transdermal drug delivery through a new wearable patch.
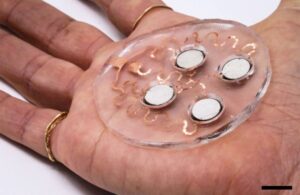
To address this, the researchers developed a wearable patch that applies painless, ultrasonic waves to the skin. This creates tiny channels that drugs can pass through, potentially enabling treatments for skin conditions. The researchers believe they can adapt the technology to deliver hormones, muscle relaxants and other drugs.
Their patch design features several disc-shaped piezoelectric transducers that convert electric currents into mechanical energy. Each disc is embedded in a polymeric cavity that contains drug molecules dissolved in a liquid solution. When the current is applied to the piezoelectric elements, it generates pressure waves in the fluid. That, in turn, makes bubbles that burst against the skin, producing microjets of fluid that can penetrate the skin’s outer layer.
The MIT team says the latest version of their device enables drugs to penetrate a few millimeters into the skin. This makes the approach useful for drugs that act locally within the skin. Those include niacinamide or vitamin C for treating age spots or other dark spots, and topical drugs used to heal burns.
Pre-filled syringes
While many of these drug delivery offerings represent potential new avenues, the well-known pre-filled syringe continues to evolve.
BD built the disinfection unit into the tip cap of the syringe. In vitro testing demonstrated a significant reduction in microbial growth associated with catheter-related bloodstream infections (CRBSIs).
Earlier this year, Schott Pharma launched its Toppac Freeze pre-fillable syringes for delivering deep-cold drugs. Deep-cold drugs are stored and transported on dry ice at temperatures reaching -100°C.
Schott designed the syringes using an advanced, pharmaceutical-grade polymer, delivered in a standardized nest-and-tub configuration. This ensures compatibility on major fill-finish lines to simplify the filling process.
Schott used cyclic olefin copolymer (COC), a break-resistant, biologically inert material that has advanced barrier properties to ensure drug stability. Its scientific data package features data on container closure integrity, functionality and particulate generation after freeze and thaw cycles.
Stevanato Group’s on-body drug delivery system
In March, Stevanato Group announced plans to bring an on-body drug delivery device to market through a collaboration with Thermo Fisher Scientific. The companies intend to offer the platform as an integrated device and fill-and-finish solution.

Stevanato Group designed Vertiva with the ability to switch between basal and bolus injections and said it is suitable for a wide range of subcutaneous therapies. The system’s single-use pod has a pre-filled, pre-loaded 3mL ISO cartridge. It also comes with a multi-use controller for potential sustainability and affordability benefits. Both parts communicate through a patented, magnetically coupled drive mechanism.
Stevanato Group said its device can adapt to different delivery profiles and enable the administration of small-molecule drugs and biologics. The company has the design under development to cover a broad range of volumes up to 10mL.

