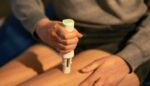
Insulet (Nasdaq:PODD) announced today that it received FDA 510(k) clearance for new enhancements to its Omnipod 5 system. The Acton, Massachusetts–based company said the updates to its tubeless, wearable automated insulin delivery patch pump’s algorithm include a new benchmark in tubeless diabetes technology with a lower, 100 mg/dL target glucose option. It also delivers a […]
FDA
FDA accepts MannKind sNDA for autoinjector that treats edema
MannKind (Nasdaq:MNKD) announced that the FDA accepted a supplemental New Drug Application (sNDA) for its Furoscix ReadyFlow autoinjector. The Furoscix ReadyFlow autoinjector (SCP-111) was developed to deliver a subcutaneous furosemide injection as an investigational alternative to the FDA-approved Furoscix on-body infusion device for treating edema in adult patients with chronic heart failure (CHF) or chronic […]
Dexcom wins FDA nod for smart CGM-integrated basal dosing for type 2 diabetes
Dexcom (NSDQ:DXCM) announced today that the FDA cleared its Smart Basal CGM-integrated basal insulin dosing optimizer. The continuous glucose monitor (CGM)-based offering received clearance for adults with type 2 diabetes on glargine U-100 long-acting insulin therapy. San Diego-based Dexcom says it marks the first and only CGM-integrated basal dosing optimizer cleared for type 2 diabetes. […]
Modular Medical submits next-gen insulin pump to FDA, expects 2026 launch
Modular Medical (Nasdaq:MODD) announced that it has submitted its next-generation Pivot insulin pump to the FDA for clearance. Modular Medical’s current pump, the MODD1, won FDA clearance nearly a year ago. It has microfluidics technology that allows for the low-cost pumping of insulin. The company announced earlier this year that it began converting its manufacturing line to […]
FDA accepts application for MannKind inhaled insulin in kids
MannKind (Nasdaq:MNKD) announced today that the FDA accepted a supplemental biologics license for its Afrezza inhaled insulin. The submission, if ultimately approved, would enable MannKind to bring Afrezza to children and adolescents (aged 4-17). Afrezza is a fast-acting insulin formulation delivered through an inhaler device. MannKind engineered the mechanical inhaler device to slowly bring powder […]
BD expands Alaris recall after identifying new potential issue
BD (NYSE:BDX) announced that it expanded its Class I voluntary recall for its Alaris Pump Module model 8100 infusion system. The expansion builds on a voluntary recall expanded earlier this year, informing customers of worst-case performance instances. Under certain cases, the pump, when used with a subset of compatible infusion sets, fails to perform as […]
FDA identifies serious recall for Dexcom CGM mobile apps
The FDA identified a recent recall of smartphone apps for Dexcom (Nasdaq:DXCM) continuous glucose monitors (CGMs) as the most serious type. This recall involves correcting certain devices through a software update. It does not involve removing them from where they are used or sold. Serious recalls can lead to serious injury or death without correction. […]
Johnson & Johnson wins FDA nod for intravesical drug delivery tech
Johnson & Johnson (NYSE:JNJ) announced today that the FDA approved Inlexzo, its gemcitabine intravesical drug delivery system. The intravesical gemcitabine-releasing system, previously known as TAR-200, treats patients with Bacillus Calmette-Guérin (BCG)-unresponsive high-risk non-muscle invasive bladder cancer (HR-NMIBC) with carcinoma in situ (CIS), with or without papillary tumors. A healthcare professional places TAR-200 into the bladder […]
Ypsomed gets FDA nod for digital add-on for autoinjectors
Ypsomed announced that the FDA granted 510(k) clearance for SmartPilot, a digital connectivity add-on for its Ypsomate autoinjectors. The device transforms the YpsoMate into a connected system that automatically captures and transmits injection data. Ypsomed said this marks an important step toward digital health solutions that offer deeper insight into therapy use. The digital add-on […]
Medtronic gets FDA nods for MiniMed 780G with Abbott Instinct sensor, expansion to type 2
Medtronic (NYSE:MDT) announced today that the FDA granted two major approvals to broaden access to its MiniMed 780G system. First, the FDA granted clearance for its SmartGuard algorithm as an interoperable automated glycemic controller (iAGC). This enables integration with a sensor made by Abbott, designed specifically through a collaboration between the medtech giants. Second, the […]






![The Inlexzo system. [Image courtesy of Johnson & Johnson]](https://www.drugdeliverybusiness.com/wp-content/uploads/2025/09/Inlexzo-JJ-2-1-150x86.jpg)