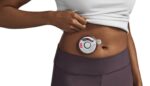
The FDA determined that the recall of the Baxter (NYSE:BAX) Novum IQ syringe pump is Class 1, the most serious kind. Baxter designed its Novum IQ syringe pump as an infusion pump to deliver fluids into a patient’s body in a controlled manner. The device is suitable for patient care in hospitals and outpatient facilities […]
FDA
FDA issues warning about over-the-counter eye drops
The FDA issued a notice warning consumers about 26 over-the-counter eye drop products that could result in eye infections. In its notice, the FDA instructed consumers not to purchase and to immediately stop using these products. Using those eye drops could lead to the risk of infections that could result in partial vision loss or […]
FDA clears iPhone app for the Insulet Omnipod 5
Insulet (Nasdaq:PODD) announced today that the FDA granted 510(k) clearance for its Omnipod 5 App for iPhone. Clearance makes Insulet the only company offering a tubeless, automated insulin delivery (AID) system with full control from a compatible Android and iOS smartphone. The company first launched the system, compatible with Android devices, in August 2022. Insulet […]
FDA approves next-gen intrathecal drug delivery system from Medtronic
Medtronic (NYSE:MDT) announced today that the FDA approved its next-generation SynchroMed III intrathecal drug delivery system. The medtech giant designed SynchroMed III to treat patients with chronic pain, cancer pain and severe spasticity. The targeted drug delivery (TDD) system alleviates symptoms by delivering medication directly to the fluid surrounding the spinal cord. SynchroMed III builds upon […]
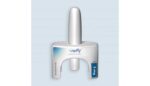
FDA approves wearable injector from Enable Injections
Enable Injections announced today that it received FDA approval for its enFuse injector for the delivery of Empaveli. Empaveli, commercialized by Apellis Pharmaceuticals, treats adults with paroxysmal nocturnal hemoglobinuria (PNH). The injector — in this case, called the Empaveli Injector — offers self-administration of the pharmaceutical. The compact, wearable device streamlines the self-administration experience with […]
FDA requests more info on neffy needle-free epinephrine
ARS Pharmaceuticals (Nasdaq:SRPY) says the FDA issued a complete response letter (CRL) regarding its neffy epinephrine nasal spray. The offering covers the emergency treatment of severe type I allergic reactions in children and adults weighing 30 kg (66 pounds) or more. The company initially set an anticipated target action date of mid-2023 for its PDUFA […]
Admetsys wins FDA breakthrough nod for automated glucose control tech
Admetsys announced today that it received FDA breakthrough device designation for its automated glucose control system. The system — also a continuous blood diagnostics system — aims to fill a key gap in the standard of care. Boston-based Admetsys designed it to automatically measure multiple blood analytes, including glucose, in real time. It requires no […]
Welldoc gets FDA nod for CGM-guided insulin bolus dosing software
Welldoc announced today that it won the 11th FDA 510(k) clearance for its BlueStar digital diabetes management platform. The clearance comes just a week after BlueStar received its 10th FDA clearance for using connected insulin dosing data in personalized bolus insulin dosing recommendations. Welldoc’s newest clearance enables BlueStar to provide bolus insulin dose recommendations based […]
BioCorRx submits implantable opioid use disorder treatment to FDA for expanded access
BioCorRx announced today that it submitted to the FDA an expanded access program application for its BICX104 implantable naltrexone pellet. BICX104, a biodegradable implantable pellet, treats opioid use disorder (OUD). The expanded access program would enable the treatment to reach eligible patients before full FDA approval. The FDA program provides access to products before approval […]
FDA clears Welldoc BlueStar for personalized bolus insulin dosing recommendations
Welldoc announced today that it received the 10th FDA 510(k) clearance for its BlueStar diabetes digital health solution. The latest regulatory nod enables BlueStar to use connected insulin dosing data in personalized bolus insulin dosing recommendations. Columbia, Maryland-based Welldoc aims to make this enhanced functionality available commercially in 2024. “The receipt of our 10th 510(k) […]