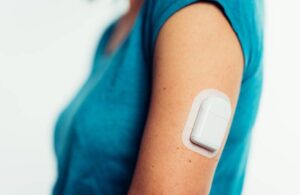
San Diego-based Tandem announced plans to acquire the maker of the Sigi patch pump for insulin delivery last month. Switzerland-based AMF Medical does not offer Sigi commercially. The system received FDA breakthrough device designation just over one year ago.
The Sigi system, an ergonomic, rechargeable patch pump, features pre-filled insulin cartridges to reduce the burden of managing diabetes.
“AMF Medical’s compelling technology aligns well with our strategic vision to include a patch pump in Tandem’s portfolio of differentiated offerings,” said John Sheridan, Tandem Diabetes Care president and CEO. “In addition to this innovative technology, the talented AMF team joining our Tandem family shares our commitment to simplify diabetes management and brings decades of experience from the diabetes device, life sciences, and watch-making industries.”
Benefits of the deal for Tandem
The company believes AMF Medical’s technology represents a “proprietary and disruptive” insulin delivery solution.
Opportunities include expanding Tandem’s type 1 and type 2 addressable market opportunities, it noted. The acquisition adds a pump meant for a patient population not addressed by Tandem today.
The deal also speeds up Tandem’s effort to bring a patch pump to market. The Sigi pump also reduces the generation of electronic and battery waste. Tandem said this enhances its sustainability objectives.
Acquiring AMF Medical marks Tandem’s second recent dip into the M&A market. The company also announced the acquisition of infusion set maker Capillary Biomedical last year.
Baker & McKenzie LLP and Bass, Berry & Sims PLC served as legal advisors to Tandem Diabetes Care. SVB Securities LLC served as the exclusive financial advisor, and Homburger AG served as the legal advisor to AMF Medical.
“AMF Medical was founded to develop a patch pump that is not only simple to use for people with diabetes, but also optimized for scale manufacturing,” said Peter Ryser, AMF Medical’s Chairman and Co-Founder. “We are thrilled that our work will be carried forward by a mission-driven company like Tandem,” added Antoine Barraud, Co-Founder and Co-CEO, noting that “Tandem not only shares our deep-rooted dedication to diabetes technology, but has created an incredibly user-focused culture that is very well aligned with our company values.”
Financial details for the acquisition
According to Tandem’s initial announcement, the acquisition includes a number of payments that include investments and milestones.
Tandem first made a strategic investment in AMF Medical in the third quarter of 2022. That investment totaled more than $8.6 million, or Swiss Francs (“CHF”) 8 million.
Tandem owed a cash payment of approximately $67.4 million (CHF 62.4 million) at closing. Additional contingent earnout payments could reach $140 million (CHF 129.6 million). That includes up to $41.5 million (CHF 38.4 million) upon successful development milestone completion over two years.
The company also agreed to pay up to $98.5 million (CHF 91.2 million) upon obtaining FDA clearance for an automated controller-enabled (ACE) pump.

