The FDA has given breakthrough device approval to a system to deliver tympanostomy tubes to young children with recurrent ear infections, in a physician’s office and under local anesthesia.
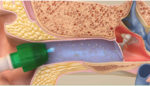
Tusker Medical’s Tubes Under Local Anesthesia (Tula) system consists of the ionic anesthetic Tymbion, Tusker Medical tympanostomy tubes, and several devices needed for the delivery of the ear tubes and the anesthetic into the ear drum.
The iontophoresis system uses a small electrical current to deliver a local anesthetic into the ear drum before tube insertion. The electrical current accelerates tissue uptake of the ionic drug, according to Tusker’s website. Tula is approved for use in both adults and children as young as six months of age.
Ear infections are common in children, with the National Institute of Deafness and other Communication Disorders (NIDCD) estimating that five of six children will have at least one ear infection before their third birthday. While health care professionals frequently prescribe antibiotics as a treatment, if antibiotics fail to treat an ear infection, or if infections continue to occur, a doctor may recommend a surgical procedure to place a small tube in the eardrum. In young children, the delivery of an ear tube has traditionally been performed in a hospital setting or surgery center and required the patient to receive general anesthesia.
The FDA said it evaluated data provided by the sponsor from 222 pediatric patients to assess the effectiveness of the Tula System for the delivery of ear tubes. The procedural success rate was 86% and 89% in children younger than age 5 and between ages 5 and 12 years old, respectively. The most common adverse event observed was inadequate anesthesia during the procedure. The Tula System should not be used in patients younger than six months of age or patients who have allergies to some local anesthetics. This product is not intended for patients who may have pre-existing issues with their eardrum, such as a perforated eardrum, the agency said.
“Today’s approval offers patients an option for the treatment of recurrent ear infections that does not require general anesthesia. As millions of children suffer from ear infections every year, it is important to have safe and effective treatments available to this susceptible patient population,” said Jeff Shuren, director of the FDA’s Center for Devices and Radiological Health, in a news release. “This approval has the potential to expand patient access to a treatment that can be administered in a physician’s office with local anesthesia and minimal discomfort.”
Menlo Park, Calif.-based Tusker Medical raised more than $10 million in 2017 to develop the system. Tusker is led by CEO Amir Abolfathi, the inventor and founder behind Sonitus Medical and its tooth-conduction hearing aid. Although the Centers for Medicare & Medicaid Services denied reimbursement for the Sonitus device, the company found a new lease on life after the U.S. military adopted the technology.

