Smith & Nephew (NYSE:SNN) today said it acquired Tusker Medical for an undisclosed amount. Tusker Medical develops the Tula System, which is an in-office ear tube delivery system to treat recurrent or persistent ear infections. The pediatric ear tubes won FDA breakthrough device designation in November last year. “The Tula System is a truly innovative option for physicians […]
Tusker Medical
Tusker Medical’s pediatric ear tubes land breakthrough device designation
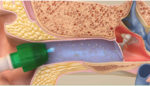
The FDA has given breakthrough device approval to a system to deliver tympanostomy tubes to young children with recurrent ear infections, in a physician’s office and under local anesthesia. Tusker Medical’s Tubes Under Local Anesthesia (Tula) system consists of the ionic anesthetic Tymbion, Tusker Medical tympanostomy tubes, and several devices needed for the delivery of […]