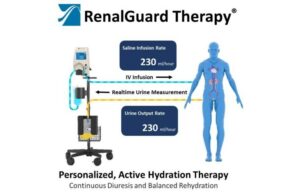
Milford, Massachusetts-based CardioRenal Systems designed the device for preventing acute kidney injury (AKI). It’s for patients at risk for cardiac surgery-associated AKI.
RenalGuard protects the kidneys with personalized, active hydration. The system maximizes urine output while balancing hydration. It does so through real-time urine output monitoring and IV infusion in a smart re-hydration system.

