Concept Medical announced today that it commenced the FDA investigational device exemption (IDE) study of its MagicTouch system. MagicTouch, a sirolimus drug-coated balloon (DCB), treats in-stent restenosis (ISR) in coronary artery disease (CAD). Dr. Said Ashraf enrolled the first patient in the MAGICAL-ISR study at the AtlantiCare Institute in Atlantic City, New Jersey. The balloon […]
Cardiovascular
Boston Scientific has positive Agent DCB study results
Boston Scientific [WtwhTicker symbol=”BSX”](NYSE: BSX)[/WtwhTicker] reported positive investigational device exemption (IDE) trial data for its Agent drug-coated balloon (DCB). The DCB just this month won FDA approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented vessel. Agent serves as […]
FDA approves Boston Scientific’s Agent drug coated balloon
Boston Scientific [WtwhTicker symbol=”BSX”](NYSE: BSX)[/WtwhTicker] announced today that it received FDA approval for its Agent drug-coated balloon (DCB). The DCB won approval for treating coronary in-stent restenosis (ISR) in patients with coronary artery disease. ISR occurs when plaque or scar tissue obstructs or narrows a stented vessel. Marlborough, Massachusetts-based Boston Scientific plans a U.S. launch for […]
Elutia submits drug-eluting biomatrix for use with cardiac implants to FDA
Elutia (Nasdaq:ELUT) announced today that it submitted a 510(k) premarket notification to the FDA for its drug-eluting biomatrix. The company designed its next-generation CanGarooRM for use with cardiac implantable electronic devices like pacemakers and defibrillators. A bioenvelope, CanGarooRM stabilizes the implantable devices. Made from a natural biomaterial that promotes a regenerative healing response, the biomatrix […]
Boston Scientific shares positive drug-coated balloon data
Boston Scientific [WtwhTicker symbol=”BSX”](NYSE: BSX)[/WtwhTicker] announced positive data from a trial of its Agent drug-coated balloon (DCB). The Marlborough, Massachusetts-based company presented its results at the Transcatheter Cardiovascular Therapeutics (TCT) meeting. Data demonstrated statistical superiority for Agent compared to uncoated balloon angioplasty. AGENT IDE evaluated the safety and effectiveness of using a DCB to treat […]
Abbott has positive data for Esprit drug-eluting resorbable scaffold
Abbott [WtwhTicker symbol=”ABT”](NYSE: ABT)[/WtwhTicker] today announced data supporting its Esprit BTK everolimus-eluting resorbable scaffold system. The company designed Esprit BTK to treat people with chronic limb-threatening ischemia (CLTI), a severe stage of peripheral artery disease (PAD). Abbott said its LIFE-BTK trial met both of its primary safety and effectiveness endpoints. It demonstrated the reduction of […]
Cordis acquires drug-eluting balloon maker MedAlliance for up to $1.135B
MedAlliance announced today that Cordis acquired it for a total consideration that could reach up to $1.135 billion. The companies initially announced the planned deal in October of last year. Cordis, which develops interventional cardiovascular and endovascular technologies, made a $35 million investment last year. Its upfront closing payment totals $200 million, with achievement milestones […]
Advanced NanoTherapies picks up $4M investment for drug-coated balloon development
Advanced NanoTherapies announced today that it collected a $4 million Series A extension from an undisclosed strategic investor. Los Gatos, California-based Advanced NanoTherapies received the investment from a medical device company. It also announced the successful treatment of the first cohort of study participants in its drug-coated balloon trial. The first-in-human trial evaluates initial short-term […]
Orchestra BioMed wins FDA IDE for drug-coated balloon study
Orchestra BioMed (Nasdaq:OBIO) announced today that the FDA granted investigational device exemption (IDE) for its Virtue SAB. The IDE enables the Virtue ISR-US pivotal study. It evaluates Virtue SAB, a novel AngioInfusion balloon for treating artery disease, in treating patients with coronary ISR. New Hope, Pennslyvania-based Orchestra BioMed designed Virtue SAB to enable the protected […]
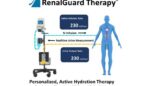
CardioRenal Systems wins FDA breakthrough nod for RenalGuard Therapy device
CardioRenal Systems announced today that it received FDA breakthrough device designation for its RenalGuard Therapy device. Milford, Massachusetts-based CardioRenal Systems designed the device for preventing acute kidney injury (AKI). It’s for patients at risk for cardiac surgery-associated AKI. RenalGuard protects the kidneys with personalized, active hydration. The system maximizes urine output while balancing hydration. It […]