Regeneron Pharmaceuticals (NSDQ:REGN) and its partner, Sanofi (NYSE:SNY), have agreed to lower the cost for its cholesterol drug, Praluent, for Express Scripts (NSDQ:ESRX) in exchange for an exclusive spot on the pharmacy benefit manager’s national formulary. The agreement, effective July 1, is designed to broaden patient access to the injectable medicine and lower out-of-pocket costs for eligible […]
Cardiovascular
How CHF Solutions is using health economics to expand product adoption
Two years after CHF Solutions (NSDQ:CHFS) acquired Baxter‘s (NYSE:BAX) Aquadex fluid filtration device for $5 million, the company’s executives are working to weave together the evidence they need to land reimbursement in the U.S. and expand patient access for its technology. The Aquadex system is designed to filter water and salt from patients experiencing fluid overload due […]
Companies push back against allegations of FCA violations over excess at-home blood testing
A 70-year-old former Marine is taking a group of companies to court over their at-home blood testing services, arguing that the industry players broke the law by requiring patients to conduct weekly tests. The companies then submit these bills to Medicare for reimbursement, even asking to be repaid for services that weren’t rendered, according to […]
FDA approves label update to Novo Nordisk’s Tresiba diabetes drug
The FDA approved Novo Nordisk‘s (NYSE:NVO) request to include heart health data on the label of its Tresiba insulin degludec product. The Danish insulin-maker evaluated its Type II diabetes drug in a 7,637-patient trial, assessing the therapy for cardiovascular safety compared to insulin glargine. Novo Nordisk’s Devote trial met its primary endpoint after Tresiba demonstrated […]
ACC ’18 Roundup: OrbusNeich Combo drug-eluting stent succeeds in registries
OrbusNeich touted pooled data from two registries evaluating its dual-therapy stent this week at the annual meeting of the American College of Cardiology. The Combo stent was implanted in more than 3,600 all-comer patients and followed by researchers for one year. The registries found that the device was safe and effective after one year, featuring a […]
BTG touts one-year follow-up data for Ekos pulmonary embolism therapy
BTG (LON:BTG) touted results today from a one-year follow-up of pulmonary embolism patients who received the company’s Ekos therapy in its Optalyse PE trial. Researchers studied the 12-month outcomes of 101 patients who were treated over a shorter period and with smaller doses of thrombolytic drugs compared to the current standard. Pulmonary embolism patients treated with BTG’s Ekos […]
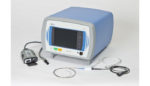
Boston Scientific’s drug-coated balloon goes up against Medtronic’s DCB in first-of-its-kind trial
Top-line results from a head-to-head trial comparing Boston Scientific‘s (NYSE:BSX) Ranger drug-coated balloon and Medtronic‘s (NYSE:MDT) In.Pact Admiral DCB found no statistically significant difference in patency rates between the two drug-device products. The trial is the first of its kind, according to Boston Scientific’s president of peripheral interventions, Jeff Mirviss. “As a leader in the […]
BioCardia wins FDA nod for chronic myocardial ischemia pivotal trial
The FDA has approved a trial to test BioCardia‘s (OTC:BCDA) CardiAMP cell therapy in chronic myocardial ischemia patients with refractory angina. The San Carlos, Calif.-based company could enroll up to 343 patients in the pivotal trial, which is designed to support benefit claims for the cell therapy product without the need for a second confirmatory […]
Medtronic’s In.Pact Admiral drug-coated balloon succeeds in two-year study, critical limb ischemia analysis
Medtronic (NYSE:MDT) touted data today from a two-year study of its In.Pact Admiral drug-coated balloon in patients with peripheral artery disease in Japan, as well as the results from a critical limb ischemia subgroup analysis from its In.Pact Global trial. The company’s In.Pact SFA Japan study enrolled 100 patients and randomized them to receive either the […]
Boehringer Ingelheim inks outcomes-based contract with pharmacy benefit manager for diabetes drug
Boehringer Ingelheim has inked an outcomes-based contract with pharmacy benefit manager Prime Therapeutics for its oral Type II diabetes drug, Jardiance. The medication is designed to cut the risk of cardiovascular death in adult patients with Type II diabetes and established heart disease. As part of Prime’s CareCentered Contracting program, the outcomes-based deal will focus […]