The FDA labeled a recall of B. Braun Medical infusion pump battery packs Class I, the most serious kind. B. Braun’s recall is a correction, not a product removal, affecting the battery pack for its Infusomat Space large-volume pump. It affects both wireless and non-wireless batteries, model numbers 8713051U and 8713052U. According to an FDA […]
Regulatory/Compliance
SiBionics wins CE mark for 14-day CGM
SiBionics announced today that it received CE mark approval in Europe for its GS1 continuous glucose monitoring (CGM) system. The company designed its CGM system to provide healthcare providers and patients with data necessary for maintaining glucose control. It offers 14 days of calibration-free continuous glucose monitoring and can transmit data to monitoring devices or […]
FDA issues warning about over-the-counter eye drops
The FDA issued a notice warning consumers about 26 over-the-counter eye drop products that could result in eye infections. In its notice, the FDA instructed consumers not to purchase and to immediately stop using these products. Using those eye drops could lead to the risk of infections that could result in partial vision loss or […]
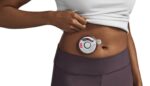
FDA clears iPhone app for the Insulet Omnipod 5
Insulet (Nasdaq:PODD) announced today that the FDA granted 510(k) clearance for its Omnipod 5 App for iPhone. Clearance makes Insulet the only company offering a tubeless, automated insulin delivery (AID) system with full control from a compatible Android and iOS smartphone. The company first launched the system, compatible with Android devices, in August 2022. Insulet […]
How the new Medtronic CGM-insulin pen combo simplifies diabetes management
Medtronic (NYSE:MDT) named its next-generation “Simplera” continuous glucose monitor (CGM) as a nod to its simplicity. The company, which just initiated the system’s launch in Europe following CE mark approval, is exceedingly optimistic about the technology. Medtronic’s all-in-one, disposable CGM features a simple, two-step insertion process for people with diabetes using multiple daily injections (MDI). Its […]
FDA approves next-gen intrathecal drug delivery system from Medtronic
Medtronic (NYSE:MDT) announced today that the FDA approved its next-generation SynchroMed III intrathecal drug delivery system. The medtech giant designed SynchroMed III to treat patients with chronic pain, cancer pain and severe spasticity. The targeted drug delivery (TDD) system alleviates symptoms by delivering medication directly to the fluid surrounding the spinal cord. SynchroMed III builds upon […]
Medtronic warns on previous software MiniMed 780G in Germany
Medtronic (NYSE:MDT) issued an urgent field safety notice in Germany to warn of a potential issue with its MiniMed 780G system. MiniMed 780G, Medtronic’s next-generation automated insulin delivery system, has been available in Europe since earning CE mark in mid-2020. In March 2021, the company first communicated that its pumps with software version 6.5 could […]
FDA approves wearable injector from Enable Injections
Enable Injections announced today that it received FDA approval for its enFuse injector for the delivery of Empaveli. Empaveli, commercialized by Apellis Pharmaceuticals, treats adults with paroxysmal nocturnal hemoglobinuria (PNH). The injector — in this case, called the Empaveli Injector — offers self-administration of the pharmaceutical. The compact, wearable device streamlines the self-administration experience with […]
Medtronic wins CE mark for next-gen Simplera CGM with InPen integration
Medtronic (NYSE:MDT) announced today that it received CE mark approval for its new Simplera continuous glucose monitor (CGM). The all-in-one, disposable CGM features a simple, two-step insertion process for people with diabetes using multiple daily injections (MDI). Medtronic’s newest no-fingerstick sensor eliminates the need for overtape, too. It seamlessly integrates with the InPen smart insulin […]
GLP-1 startup i2o looks to revive diabetes drug-eluting implant from Intarcia
i2o Therapeutics says it acquired the diabetes-treating assets of one-time medtech unicorn Intarcia Therapeutics. The Boston-based company acquired and integrated Intarcia’s proprietary assets and made a big personnel move. With CEO Ravi Srinivasan moving onto other opportunities, i2o named Kurt Graves as chair, president and CEO. Graves previously served as executive chair of the i2o […]