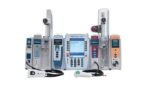
BD (NYSE:BDX) today voluntarily posted a product security bulletin for a number of vulnerabilities with its Alaris infusion system. Franklin Lakes, New Jersey-based BD recently identified eight vulnerabilities. These vulnerabilities are associated with the BD Alaris system with Guardrails Suite MX, versions 12.1.3 and earlier. The company discovered the vulnerabilities through routine internal security testing […]
Regulatory/Compliance
FDA approves drug-coated balloon for BPH symptoms from Urotronic
Urotronic announced that the FDA approved its Optilume BPH catheter system for to alleviate urinary symptoms caused by BPH. Minneapolis-based Urotronic designed Optilume as a minimally invasive surgical therapy. It combines mechanical dilation using a proprietary double-lobe balloon with concurrent localized delivery of paclitaxel. This treats lower urinary tract symptoms secondary to BPG (benign prostatic […]
FDA updates guidance on paclitaxel-coated devices, determines no link to late mortality risk
The FDA issued healthcare providers updated guidance for certain warning language with paclitaxel-coated devices that treat PAD. These peripheral arterial disease (PAD)-treating devices produced data that does not support an excess mortality risk. Specifically, the FDA’s guidance eliminates the need for certain warning language in the device labeling. An FDA panel in 2019 determined that […]
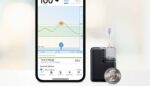
FDA clears Tandem Diabetes Care’s Mobi durable automated insulin pump
Tandem Diabetes Care (Nasdaq:TNDM) announced today that the FDA cleared its Tandem Mobi automated insulin delivery (AID) system. Clearance covers people with diabetes ages six and up, expanding the Tandem portfolio of products. San Diego-based Tandem says Mobi, which is fully controllable from a mobile app, is the world’s smallest durable AID system. Mobi features […]
Koru Medical submits infusion system for FDA 510(k) clearance
Koru Medical Systems (Nasdaq:KRMD) announced today that it submitted its Freedom60 infusion system for FDA 510(k) clearance. The submission covers the use of the Freedom60 infusion system with the Hizentra 50 mL prefilled syringe. In January, Koru inked a development agreement with a manufacturer of subcutaneous immunoglobulin therapy (SCIg). The deal sought to garner regulatory […]
Medicare now covers new Medtronic MiniMed 780G automated insulin pump
Medtronic (NYSE:MDT) announced today that Medicare now covers its new MiniMed 780G system with the Guardian 4 sensor. Coverage for the recently FDA-approved automated insulin delivery system extends to all eligible Medicare and Medicare Advantage beneficiaries. Medtronic intends to begin processing orders immediately. The company plans to start shipments to eligible recipients with type 1 […]
CellTrans wins FDA approval for first cell therapy that treats type 1 diabetes
The FDA announced that it approved the Lantidra allogeneic pancreatic islet cellular therapy developed by CellTrans to treat diabetes. It marks the first approval of a donor pancreatic cellular therapy — made from deceased donor pancreatic cells — to treat type 1 diabetes. The approval covers adults with type 1 diabetes who prove unable to […]
France expands reimbursement for Abbott FreeStyle Libre 2 to all basal insulin users
Abbott (NYSE:ABT) announced today that the French health authority approved the expansion of reimbursement coverage for the FreeStyle Libre 2. FreeStyle Libre 2 previously only had coverage for people with type 1 and type 2 diabetes who require intensive insulin therapy. France’s expansion covers the continuous glucose monitor (CGM) for all people who use basal […]
FDA approves Surmodics SurVeil drug-coated balloon
Surmodics (Nasdaq:SRDX) announced today that the FDA granted approval for its SurVeil drug-coated balloon (DCB). Eden Prairie, Minnesota-based Surmodis may now market and sell SurVeil in the U.S. for percutaneous transluminal angioplasty. Use follows appropriate vessel preparation or de novo or restenotic lesions in femoral and popliteal arteries. These arteries feature reference vessel diameters of […]
ICU Medical recalls certain infusion pump replacement batteries
The FDA issued a notice determining that the recall of ICU Medical (Nasdaq:ICUI) infusion pump batteries is Class I, the most serious kind. ICU Medical initiated a recall of replacement batteries for certain infusion systems on March 22, 2023. The recall affects its Plum 360, Plum A+ and Plum A+3 infusion systems. The infusion systems […]