Delcath Systems (NSDQ:DCTH) filed a new FDA application for the latest iteration of its ChemoSat drug-device combination, the Generation 2 device, in treatment of patients with non-operable liver cancer.
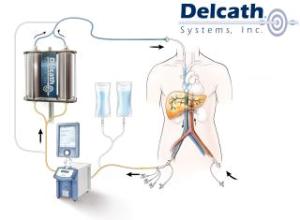
Delcath’s ChemoSat uses a system of tubes to localize the delivery of toxic chemotherapy agents by isolating the targeted organ — in this case, the liver — from the rest of the body’s bloodstream.
Delcath must seek new drug approval for the melphalan hydrochloride the ChemoSat system uses to destroy cancerous tissue.
"Our team has achieved a significant milestone with the filing of our NDA," president & CEO Eamonn Hobbs said in prepared remarks. "Based upon the strength of our Phase I, II and III data, along with the limited treatment options available for patients with unresectable melanoma metastases in the liver, we believe that our application meets the FDA’s criteria for priority review."
The FDA refused Delcath’s original NDA, filed last year, over concerns that the information provided was insufficient for review.
Check out our interview with Delcath president & CEO Eamonn Hobbs.
The watchdog agency’s response came as a surprise, Hobbs told MassDevice.com last year, as clinical trials had been designed and conducted under a special FDA agreement.
"We conducted our Phase III trial under an SPA, a special protocol assessment, which is a formal agreement with the agency on all the facts and circumstances associated with the trial," Hobbs said. "They wanted additional hospital data that was not collected in the original case report forms that were approved as part of the SPA."
Delcath re-mined it clinical data for the information the FDA had asked for in preparation for a pre-review meeting for a 2nd round with the FDA.
The federal watchdog agency had previously granted Delcath 2 orphan drug designations for treatment in ocular and cutaneous melanoma, which will give the company exclusivity in those indications for 7 years if the NDA is approved.

